Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age
- PMID: 26010764
- PMCID: PMC4933107
- DOI: 10.1113/JP270710
Depressed perivascular sensory innervation of mouse mesenteric arteries with advanced age
Abstract
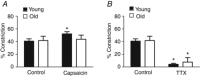
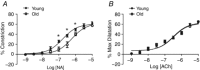
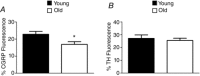
Key points: The dilatory role for sensory innervation of mesenteric arteries (MAs) is impaired in Old (∼24 months) versus Young (∼4 months) mice. We investigated the nature of this impairment in isolated pressurized MAs. With perivascular sensory nerve stimulation, dilatation and inhibition of sympathetic vasoconstriction observed in Young MAs were lost in Old MAs along with impaired dilatation to calcitonin gene-related peptide (CGRP). Inhibiting NO and prostaglandin synthesis increased CGRP EC50 in Young and Old MAs. Endothelial denudation attenuated dilatation to CGRP in Old MAs yet enhanced dilatation to CGRP in Young MAs while abolishing all dilatations to ACh. In Old MAs, sensory nerve density was reduced and RAMP1 (CGRP receptor component) associated with nuclear regions of endothelial cells in a manner not seen in Young MAs or in smooth muscle cells of either age. With advanced age, loss of dilatory signalling mediated through perivascular sensory nerves may compromise perfusion of visceral organs.
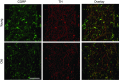
Abstract: Vascular dysfunction and sympathetic nerve activity increase with advancing age. In the gut, blood flow is governed by perivascular sensory and sympathetic nerves but little is known of how their functional role is affected by advanced age. We tested the hypothesis that functional sensory innervation of mesenteric arteries (MAs) is impaired for Old (24 months) versus Young (4 months) C57BL/6 male mice. In cannulated pressurized MAs preconstricted 50% with noradrenaline and treated with guanethidine (to inhibit sympathetic neurotransmission), perivascular nerve stimulation (PNS) evoked dilatation in Young but not Old MAs while dilatations to ACh were not different between age groups. In Young MAs, capsaicin (to inhibit sensory neurotransmission) blocked dilatation and increased constriction during PNS. With no difference in efficacy, the EC50 of CGRP as a vasodilator was ∼6-fold greater in Old versus Young MAs. Inhibiting nitric oxide (l-NAME) and prostaglandin (indomethacin) synthesis increased CGRP EC50 in both age groups. Endothelial denudation reduced the efficacy of dilatation to CGRP by ∼30% in Old MAs yet increased this efficacy ∼15% in Young MAs while all dilatations to ACh were abolished. Immunolabelling revealed reduced density of sensory (CGRP) but not sympathetic (tyrosine hydroxylase) innervation for Old versus Young MAs. Whereas the distribution of CGRP receptor proteins was similar in SMCs, RAMP1 associated with nuclear regions of endothelial cells of Old but not Young MAs. With advanced age, the loss of sensory nerve function and diminished effectiveness of CGRP as a vasodilator is multifaceted and may adversely affect splanchnic perfusion.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



References
-
- Abramoff MD, Magelhaes PJ & Ram SJ (2004). Image processing with ImageJ. Biophoton Int 11, 36–42.
-
- Amerini S, Mantelli L, Filippi S & Ledda F (1994). Effects of aging and hypertension on vasorelaxant activity of calcitonin gene‐related peptide: a comparison with other vasodilator agents. J Cardiovasc Pharmacol 23, 432–437. - PubMed
-
- Armstead WM (1995). Role of nitric oxide and cAMP in prostaglandin‐induced pial arterial vasodilation. Am J Physiol Heart Circ Physiol 268, H1436–1440. - PubMed
-
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S & Luscher TF (1997). Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension 30, 817–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

