Hyperbaric oxygen therapy can diminish fibromyalgia syndrome--prospective clinical trial
- PMID: 26010952
- PMCID: PMC4444341
- DOI: 10.1371/journal.pone.0127012
Hyperbaric oxygen therapy can diminish fibromyalgia syndrome--prospective clinical trial
Abstract
Background: Fibromyalgia Syndrome (FMS) is a persistent and debilitating disorder estimated to impair the quality of life of 2-4% of the population, with 9:1 female-to-male incidence ratio. FMS is an important representative example of central nervous system sensitization and is associated with abnormal brain activity. Key symptoms include chronic widespread pain, allodynia and diffuse tenderness, along with fatigue and sleep disturbance. The syndrome is still elusive and refractory. The goal of this study was to evaluate the effect of hyperbaric oxygen therapy (HBOT) on symptoms and brain activity in FMS.
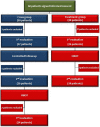
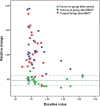
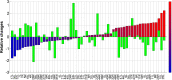
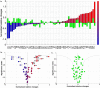
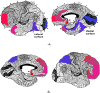
Methods and findings: A prospective, active control, crossover clinical trial. Patients were randomly assigned to treated and crossover groups: The treated group patients were evaluated at baseline and after HBOT. Patients in the crossover-control group were evaluated three times: baseline, after a control period of no treatment, and after HBOT. Evaluations consisted of physical examination, including tender point count and pain threshold, extensive evaluation of quality of life, and single photon emission computed tomography (SPECT) imaging for evaluation of brain activity. The HBOT protocol comprised 40 sessions, 5 days/week, 90 minutes, 100% oxygen at 2ATA. Sixty female patients were included, aged 21-67 years and diagnosed with FMS at least 2 years earlier. HBOT in both groups led to significant amelioration of all FMS symptoms, with significant improvement in life quality. Analysis of SPECT imaging revealed rectification of the abnormal brain activity: decrease of the hyperactivity mainly in the posterior region and elevation of the reduced activity mainly in frontal areas. No improvement in any of the parameters was observed following the control period.
Conclusions: The study provides evidence that HBOT can improve the symptoms and life quality of FMS patients. Moreover, it shows that HBOT can induce neuroplasticity and significantly rectify abnormal brain activity in pain related areas of FMS patients.
Trial registration: ClinicalTrials.gov NCT01827683.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

