Replication stress in Mammalian cells and its consequences for mitosis
- PMID: 26010955
- PMCID: PMC4488665
- DOI: 10.3390/genes6020267
Replication stress in Mammalian cells and its consequences for mitosis
Abstract
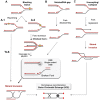
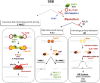
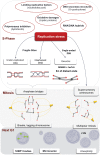
The faithful transmission of genetic information to daughter cells is central to maintaining genomic stability and relies on the accurate and complete duplication of genetic material during each cell cycle. However, the genome is routinely exposed to endogenous and exogenous stresses that can impede the progression of replication. Such replication stress can be an early cause of cancer or initiate senescence. Replication stress, which primarily occurs during S phase, results in consequences during mitosis, jeopardizing chromosome segregation and, in turn, genomic stability. The traces of replication stress can be detected in the daughter cells during G1 phase. Alterations in mitosis occur in two types: 1) local alterations that correspond to breaks, rearrangements, intertwined DNA molecules or non-separated sister chromatids that are confined to the region of the replication dysfunction; 2) genome-wide chromosome segregation resulting from centrosome amplification (although centrosomes do not contain DNA), which amplifies the local replication stress to the entire genome. Here, we discuss the endogenous causes of replication perturbations, the mechanisms of replication fork restart and the consequences for mitosis, chromosome segregation and genomic stability.
Keywords: anaphase bridges; centrosome; fragile sites; homologous recombination; micronuclei; mitosis; replication stress; single-ended DSB.
Figures


References
-
- Gorgoulis V.G., Vassiliou L.F., Karakaidos P., Zacharatos P., Kotsinas A., Liloglou T., Venere M., Ditullio R.A., Jr., Kastrinakis N.G., Levy B., et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. - DOI - PubMed
-
- Flach J., Bakker S.T., Mohrin M., Conroy P.C., Pietras E.M., Reynaud D., Alvarez S., Diolaiti M.E., Ugarte F., Forsberg E.C., et al. Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature. 2014;512:198–202. doi: 10.1038/nature13619. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

