Review article: dietary fibre-microbiota interactions
- PMID: 26011307
- PMCID: PMC4949558
- DOI: 10.1111/apt.13248
Review article: dietary fibre-microbiota interactions
Abstract
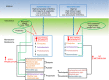
Background: Application of modern rapid DNA sequencing technology has transformed our understanding of the gut microbiota. Diet, in particular plant-based fibre, appears critical in influencing the composition and metabolic activity of the microbiome, determining levels of short-chain fatty acids (SCFAs) important for intestinal health.
Aim: To assess current epidemiological, experimental and clinical evidence of how long-term and short-term alterations in dietary fibre intake impact on the microbiome and metabolome.
Methods: A Medline search including items 'intestinal microbiota', 'nutrition', 'diet', 'dietary fibre', 'SCFAs' and 'prebiotic effect' was performed.
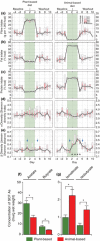
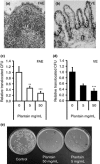
Results: Studies found evidence of fibre-influenced differences in the microbiome and metabolome as a consequence of habitual diet, and of long-term or short-term intervention (in both animals and humans).
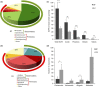
Conclusions: Agrarian diets high in fruit/legume fibre are associated with greater microbial diversity and a predominance of Prevotella over Bacteroides. 'Western'-style diets, high in fat/sugar, low in fibre, decrease beneficial Firmicutes that metabolise dietary plant-derived polysaccharides to SCFAs and increase mucosa-associated Proteobacteria (including enteric pathogens). Short-term diets can also have major effects, particularly those exclusively animal-based, and those high-protein, low-fermentable carbohydrate/fibre 'weight-loss' diets, increasing the abundance of Bacteroides and lowering Firmicutes, with long-term adherence to such diets likely increasing risk of colonic disease. Interventions to prevent intestinal inflammation may be achieved with fermentable prebiotic fibres that enhance beneficial Bifidobacteria or with soluble fibres that block bacterial-epithelial adherence (contrabiotics). These mechanisms may explain many of the differences in microbiota associated with long-term ingestion of a diet rich in fruit and vegetable fibre.
© 2015 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

References
-
- Hold GL. The gut microbiota, dietary extremes and exercise. Gut 2014; 63: 1838–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
