Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial
- PMID: 26011677
- PMCID: PMC5033065
- DOI: 10.1002/ejhf.300
Maintenance of serum potassium with sodium zirconium cyclosilicate (ZS-9) in heart failure patients: results from a phase 3 randomized, double-blind, placebo-controlled trial
Abstract
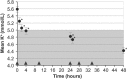
Aims: Hyperkalaemia in heart failure patients limits use of cardioprotective renin-angiotensin-aldosterone system inhibitors (RAASi). Sodium zirconium cyclosilicate (ZS-9) is a selective potassium ion trap, whose mechanism of action may allow for potassium binding in the upper gastrointestinal tract as early as the duodenum following oral administration. ZS-9 previously demonstrated the ability to reduce elevated potassium levels into the normal range, with a median time of normalization of 2.2 h and sustain normal potassium levels for 28 days in HARMONIZE--a Phase 3, double-blind, randomized, placebo-controlled trial. In the present study we evaluated management of serum potassium with daily ZS-9 over 28 days in heart failure patients from HARMONIZE, including those receiving RAASi therapies.
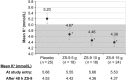
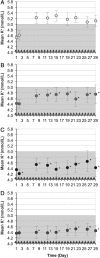
Methods and results: Heart failure patients with evidence of hyperkalaemia (serum potassium ≥5.1 mmol/L, n = 94) were treated with open-label ZS-9 for 48 h. Patients (n = 87; 60 receiving RAASi) who achieved normokalaemia (potassium 3.5-5.0 mmol/L) were randomized to daily ZS-9 (5, 10, or 15 g) or placebo for 28 days. Mean potassium and proportion of patients maintaining normokalaemia during days 8-29 post-randomization were evaluated. Despite RAASi doses being kept constant, patients on 5 g, 10 g, and 15 g ZS-9 maintained a lower potassium level (4.7 mmol/L, 4.5 mmol/L, and 4.4 mmol/L, respectively) than the placebo group (5.2 mmol/L; P<0.01 vs. each ZS-9 group); greater proportions of ZS-9 patients (83%, 89%, and 92%, respectively) maintained normokalaemia than placebo (40%; P < 0.01 vs. each ZS-9 group). The safety profile was consistent with previously reported overall study population.
Conclusion: Compared with placebo, all three ZS-9 doses lowered potassium and effectively maintained normokalaemia for 28 days in heart failure patients without adjusting concomitant RAASi, while maintaining a safety profile consistent with the overall study population.
Keywords: RAAS; ZS-9; heart failure; hyperkalaemia.
© 2015 The Authors European Journal of Heart Failure © 2015 European Society of Cardiology.
Figures

Comment in
-
Hyperkalaemia in heart failure: binding the patient to improved treatment?Eur J Heart Fail. 2015 Oct;17(10):997-9. doi: 10.1002/ejhf.404. Epub 2015 Oct 7. Eur J Heart Fail. 2015. PMID: 26443125 No abstract available.
References
-
- Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, Reilly RF, Banerjee S. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 2012;109:1510–1513. - PubMed
-
- Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail 2004;10:297–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

