Human hemorrhagic Fever causing arenaviruses: molecular mechanisms contributing to virus virulence and disease pathogenesis
- PMID: 26011826
- PMCID: PMC4493475
- DOI: 10.3390/pathogens4020283
Human hemorrhagic Fever causing arenaviruses: molecular mechanisms contributing to virus virulence and disease pathogenesis
Abstract
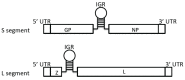
Arenaviruses include multiple human pathogens ranging from the low-risk lymphocytic choriomeningitis virus (LCMV) to highly virulent hemorrhagic fever (HF) causing viruses such as Lassa (LASV), Junin (JUNV), Machupo (MACV), Lujo (LUJV), Sabia (SABV), Guanarito (GTOV), and Chapare (CHPV), for which there are limited preventative and therapeutic measures. Why some arenaviruses can cause virulent human infections while others cannot, even though they are isolated from the same rodent hosts, is an enigma. Recent studies have revealed several potential pathogenic mechanisms of arenaviruses, including factors that increase viral replication capacity and suppress host innate immunity, which leads to high viremia and generalized immune suppression as the hallmarks of severe and lethal arenaviral HF diseases. This review summarizes current knowledge of the roles of each of the four viral proteins and some known cellular factors in the pathogenesis of arenaviral HF as well as of some human primary cell-culture and animal models that lend themselves to studying arenavirus-induced HF disease pathogenesis. Knowledge gained from these studies can be applied towards the development of novel therapeutics and vaccines against these deadly human pathogens.
Keywords: Junin virus; Lassa virus; arenaviruses; immune evasion; pathogenic mechanisms; virus replication.
Figures

References
-
- Maiztegui J.I., McKee K.T., Oro J.G.B., Harrison L.H., Gibbs P.H., Feuillade M.R., Enria D.A., Briggiler A.M., Levis S.C., Ambrosio A.M., et al. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. J. Infect. Dis. 1998;177:277–283. doi: 10.1086/514211. - DOI - PubMed
-
- Radoshitzky S.R., Bao Y., Buchmeier M.J., Charrel R.N., Clawson A.N., Clegg C.S., DeRisi J.L., Emonet S., Gonzalez J.P., Kuhn J.H., et al. Past, present, and future of arenavirus taxonomy. Arch. Virol. 2015 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

