Bile duct-ligated mice exhibit multiple phenotypic similarities to acute decompensation patients despite histological differences
- PMID: 26012885
- PMCID: PMC4869675
- DOI: 10.1111/liv.12876
Bile duct-ligated mice exhibit multiple phenotypic similarities to acute decompensation patients despite histological differences
Abstract
Background & aims: Patients with decompensated cirrhosis are susceptible to infection. Innate immune dysfunction and development of organ failure are considered to underlie this. A rodent model of liver disease sharing these phenotypic features would assist in vivo study of underlying mechanisms and testing of therapeutics. We evaluated three models to identify which demonstrated the greatest clinical and immunological phenotypic similarity to patients with acutely decompensated (AD) cirrhosis.
Methods: We selected Bile Duct Ligation (BDL) rats at 4 weeks, BDL mice at 14 days and Carbon tetrachloride (CCl4 ) mice at 10 weeks (with studies performed 7 days after final CCl4 infection). We examined organ dysfunction, inflammatory response to carrageenan-in-paw, plasma eicosanoid concentrations, macrophage cytokine production and responses to peritoneal infection.
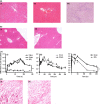
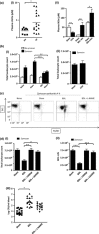
Results: Bile duct ligation caused sarcopenia, liver, cardiovascular and renal dysfunction whereas CCl4 mice demonstrated no clinical abnormalities. BDL rodents exhibited depressed response to carrageenan-in-paw unlike CCl4 mice. BDL rats have slightly elevated plasma eicosanoid levels and plasma showed partial PGE2 -mediated immune suppression whereas CCl4 mice did not. Plasma NOx was elevated in patients with acute or chronic liver failure (AoCLF) compared to healthy volunteers and BDL rodents but not CCl4 mice. Elevated nitric oxide (NO) via inducible nitric oxide synthase (iNOS) mediates defective leucocyte trafficking in BDL rodent models.
Conclusions: We conclude that BDL mice and rats are not simply models of cholestatic liver injury but may be used to study mechanisms underlying poor outcome from infection in AD and have identified elevated NO as a potential mediator of depressed leucocyte trafficking.
Keywords: bile duct ligation; carbon tetrachloride; eicosanoids; immune suppression; leucocyte trafficking.
© 2015 The Authors. Liver International Published by John Wiley & Sons Ltd.
Figures

References
-
- Blachier M, Leleu H, Peck‐Radosavljevic M, Valla DC, Roudot‐Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol 2013; 58: 593–608. - PubMed
-
- Zatonski WA, Sulkowska U, Manczuk M, et al Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe. Eur Addict Res 2010; 16: 193–201. - PubMed
-
- Navasa M, Fernandez J, Rodes J. Bacterial infections in liver cirrhosis. Ital J Gastroenterol Hepatol 1999; 31: 616–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

