miR-375 gene dosage in pancreatic β-cells: implications for regulation of β-cell mass and biomarker development
- PMID: 26013143
- PMCID: PMC4589563
- DOI: 10.1007/s00109-015-1296-9
miR-375 gene dosage in pancreatic β-cells: implications for regulation of β-cell mass and biomarker development
Abstract
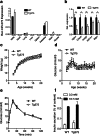
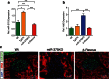
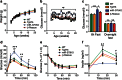
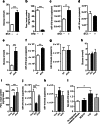
MicroRNAs play a crucial role in the regulation of cell growth and differentiation. Mice with genetic deletion of miR-375 exhibit impaired glycemic control due to decreased β-cell and increased α-cell mass and function. The relative importance of these processes for the overall phenotype of miR-375KO mice is unknown. Here, we show that mice overexpressing miR-375 exhibit normal β-cell mass and function. Selective re-expression of miR-375 in β-cells of miR-375KO mice normalizes both, α- and β-cell phenotypes as well as glucose metabolism. Using this model, we also analyzed the contribution of β-cells to the total plasma miR-375 levels. Only a small proportion (≈1 %) of circulating miR-375 originates from β-cells. Furthermore, acute and profound β-cell destruction is sufficient to detect elevations of miR-375 levels in the blood. These findings are supported by higher miR-375 levels in the circulation of type 1 diabetes (T1D) subjects but not mature onset diabetes of the young (MODY) and type 2 diabetes (T2D) patients. Together, our data support an essential role for miR-375 in the maintenance of β-cell mass and provide in vivo evidence for release of miRNAs from pancreatic β-cells. The small contribution of β-cells to total plasma miR-375 levels make this miRNA an unlikely biomarker for β-cell function but suggests a utility for the detection of acute β-cell death for autoimmune diabetes.
Key messages: • Overexpression of miR-375 in β-cells does not influence β-cell mass and function. • Increased α-cell mass in miR-375KO arises secondarily to loss of miR-375 in β-cells. • Only a small proportion of circulating miR-375 levels originates from β-cells. • Acute β-cell destruction results in measurable increases of miR-375 in the blood. Circulating miR-375 levels are not a biomarker for pancreatic β-cell function.
Keywords: Biomarker; Diabetes; MiRNA-375; Pancreatic β-cells; β-cell mass.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

