Structure of the tripartite multidrug efflux pump AcrAB-TolC suggests an alternative assembly mode
- PMID: 26013259
- PMCID: PMC4332038
- DOI: 10.14348/molcells.2015.2277
Structure of the tripartite multidrug efflux pump AcrAB-TolC suggests an alternative assembly mode
Abstract
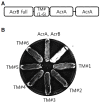
Escherichia coli AcrAB-TolC is a multidrug efflux pump that expels a wide range of toxic substrates. The dynamic nature of the binding or low affinity between the components has impeded elucidation of how the three components assemble in the functional state. Here, we created fusion proteins composed of AcrB, a transmembrane linker, and two copies of AcrA. The fusion protein exhibited acridine pumping activity, suggesting that the protein reflects the functional structure in vivo. To discern the assembling mode with TolC, the AcrBA fusion protein was incubated with TolC or a chimeric protein containing the TolC aperture tip region. Three-dimensional structures of the complex proteins were determined through transmission electron microscopy. The overall structure exemplifies the adaptor bridging model, wherein the funnel-like AcrA hexamer forms an intermeshing cogwheel interaction with the α-barrel tip region of TolC, and a direct interaction between AcrB and TolC is not allowed. These observations provide a structural blueprint for understanding multidrug resistance in pathogenic Gram-negative bacteria.
Keywords: complex structure; electron microscopy; membrane protein; multidrug efflux pump.
Figures



Similar articles
-
Computer simulations suggest direct and stable tip to tip interaction between the outer membrane channel TolC and the isolated docking domain of the multidrug RND efflux transporter AcrB.Biochim Biophys Acta. 2016 Jul;1858(7 Pt A):1419-26. doi: 10.1016/j.bbamem.2016.03.029. Epub 2016 Apr 2. Biochim Biophys Acta. 2016. PMID: 27045078
-
The C-terminal domain of AcrA is essential for the assembly and function of the multidrug efflux pump AcrAB-TolC.J Bacteriol. 2009 Jul;191(13):4365-71. doi: 10.1128/JB.00204-09. Epub 2009 May 1. J Bacteriol. 2009. PMID: 19411330 Free PMC article.
-
In situ structure of the AcrAB-TolC efflux pump at subnanometer resolution.Structure. 2022 Jan 6;30(1):107-113.e3. doi: 10.1016/j.str.2021.08.008. Epub 2021 Sep 9. Structure. 2022. PMID: 34506732 Free PMC article.
-
AcrAB and related multidrug efflux pumps of Escherichia coli.J Mol Microbiol Biotechnol. 2001 Apr;3(2):215-8. J Mol Microbiol Biotechnol. 2001. PMID: 11321576 Review.
-
The AcrB efflux pump: conformational cycling and peristalsis lead to multidrug resistance.Curr Drug Targets. 2008 Sep;9(9):729-49. doi: 10.2174/138945008785747789. Curr Drug Targets. 2008. PMID: 18781920 Review.
Cited by
-
Structural basis of RND-type multidrug exporters.Front Microbiol. 2015 Apr 20;6:327. doi: 10.3389/fmicb.2015.00327. eCollection 2015. Front Microbiol. 2015. PMID: 25941524 Free PMC article. Review.
-
An allosteric transport mechanism for the AcrAB-TolC multidrug efflux pump.Elife. 2017 Mar 29;6:e24905. doi: 10.7554/eLife.24905. Elife. 2017. PMID: 28355133 Free PMC article.
-
Using a SMALP platform to determine a sub-nm single particle cryo-EM membrane protein structure.Biochim Biophys Acta Biomembr. 2018 Feb;1860(2):378-383. doi: 10.1016/j.bbamem.2017.10.005. Epub 2017 Oct 6. Biochim Biophys Acta Biomembr. 2018. PMID: 28993151 Free PMC article.
-
Mg2+-dependent mechanism of environmental versatility in a multidrug efflux pump.bioRxiv [Preprint]. 2024 Jun 10:2024.06.10.597921. doi: 10.1101/2024.06.10.597921. bioRxiv. 2024. Update in: Structure. 2025 Mar 06;33(3):552-565.e4. doi: 10.1016/j.str.2024.12.012. PMID: 38915626 Free PMC article. Updated. Preprint.
-
The ins and outs of RND efflux pumps in Escherichia coli.Front Microbiol. 2015 Jun 10;6:587. doi: 10.3389/fmicb.2015.00587. eCollection 2015. Front Microbiol. 2015. PMID: 26113845 Free PMC article. Review.
References
-
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. - PubMed
-
- Goddard TD, Huang CC, Ferrin TE. Visualizing density maps with UCSF Chimera. J Struct Biol. 2007;157:281–287. - PubMed
-
- Hinchliffe P, Symmons MF, Hughes C, Koronakis V. Structure and operation of bacterial tripartite pumps. Annu Rev Microbiol. 2013;67:221–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

