Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases
- PMID: 26013557
- PMCID: PMC4444976
- DOI: 10.1136/bmj.h2135
Use of combined oral contraceptives and risk of venous thromboembolism: nested case-control studies using the QResearch and CPRD databases
Abstract
Objective: To investigate the association between use of combined oral contraceptives and risk of venous thromboembolism, taking the type of progestogen into account.
Design: Two nested case-control studies.
Setting: General practices in the United Kingdom contributing to the Clinical Practice Research Datalink (CPRD; 618 practices) and QResearch primary care database (722 practices).
Participants: Women aged 15-49 years with a first diagnosis of venous thromboembolism in 2001-13, each matched with up to five controls by age, practice, and calendar year.
Main outcome measures: Odds ratios for incident venous thromboembolism and use of combined oral contraceptives in the previous year, adjusted for smoking status, alcohol consumption, ethnic group, body mass index, comorbidities, and other contraceptive drugs. Results were combined across the two datasets.
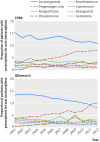
Results: 5062 cases of venous thromboembolism from CPRD and 5500 from QResearch were analysed. Current exposure to any combined oral contraceptive was associated with an increased risk of venous thromboembolism (adjusted odds ratio 2.97, 95% confidence interval 2.78 to 3.17) compared with no exposure in the previous year. Corresponding risks associated with current exposure to desogestrel (4.28, 3.66 to 5.01), gestodene (3.64, 3.00 to 4.43), drospirenone (4.12, 3.43 to 4.96), and cyproterone (4.27, 3.57 to 5.11) were significantly higher than those for second generation contraceptives levonorgestrel (2.38, 2.18 to 2.59) and norethisterone (2.56, 2.15 to 3.06), and for norgestimate (2.53, 2.17 to 2.96). The number of extra cases of venous thromboembolism per year per 10,000 treated women was lowest for levonorgestrel (6, 95% confidence interval 5 to 7) and norgestimate (6, 5 to 8), and highest for desogestrel (14, 11 to 17) and cyproterone (14, 11 to 17).
Conclusions: In these population based, case-control studies using two large primary care databases, risks of venous thromboembolism associated with combined oral contraceptives were, with the exception of norgestimate, higher for newer drug preparations than for second generation drugs.
© Vinogradova et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


Comment in
-
Fresh evidence confirms links between newer contraceptive pills and higher risk of venous thromboembolism.BMJ. 2015 May 26;350:h2422. doi: 10.1136/bmj.h2422. BMJ. 2015. PMID: 26013974 No abstract available.
-
New study supports MHRA advice on combined oral contraceptives.BMJ. 2015 Jun 24;350:h3305. doi: 10.1136/bmj.h3305. BMJ. 2015. PMID: 26108434 No abstract available.
-
Oral contraceptive study omitted several confounders.BMJ. 2015 Jun 24;350:h3303. doi: 10.1136/bmj.h3303. BMJ. 2015. PMID: 26108435 No abstract available.
-
Authors' reply to Zagarella.BMJ. 2015 Jun 24;350:h3308. doi: 10.1136/bmj.h3308. BMJ. 2015. PMID: 26109585 No abstract available.
-
Combined oral contraceptives and risk of venous thromboembolism: there is higher risk in new generations compared to second generations, but paradoxically not in norgestimate-containing-pills.Evid Based Med. 2015 Oct;20(5):189. doi: 10.1136/ebmed-2015-110249. Epub 2015 Aug 12. Evid Based Med. 2015. PMID: 26269361 No abstract available.
-
Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study.BMJ. 2016 May 10;353:i2002. doi: 10.1136/bmj.i2002. BMJ. 2016. PMID: 27164970 Free PMC article.
References
-
- Department of Economic and Social Affairs. World contraceptive patterns 2013. United Nations, 2013.
-
- Lewis M, Heinemann L, MacRae K, Bruppacher R, Spitzer W. The increased risk of venous thromboembolism and the use of third generation progestagens: role of bias in observational research. Contraception 1996;54:5-13. - PubMed
-
- Farmer RDT, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997;349:83-8. - PubMed
-
- Lawrenson R, Farmer R. Venous thromboembolism and combined oral contraceptives: does the type of progestogen make a difference? Contraception 2000;62(2 suppl):S21-8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
