Osteonecrosis of the Jaw Developed in Mice: DISEASE VARIANTS REGULATED BY γδ T CELLS IN ORAL MUCOSAL BARRIER IMMUNITY
- PMID: 26013832
- PMCID: PMC4498073
- DOI: 10.1074/jbc.M115.652305
Osteonecrosis of the Jaw Developed in Mice: DISEASE VARIANTS REGULATED BY γδ T CELLS IN ORAL MUCOSAL BARRIER IMMUNITY
Abstract
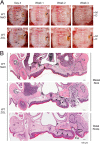
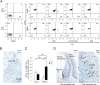
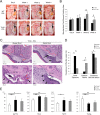
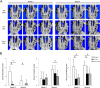
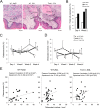
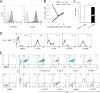
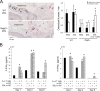
Osteonecrosis of the jaw (ONJ), an uncommon co-morbidity in patients treated with bisphosphonates (BP), occurs in the segment of jawbone interfacing oral mucosa. This study aimed to investigate a role of oral mucosal barrier γδ T cells in the pathogenesis of ONJ. Female C57Bl/6J (B6) mice received a bolus zoledronate intravenous injection (ZOL, 540 μg/kg), and their maxillary left first molars were extracted 1 week later. ZOL-treated mice (WT ZOL) delayed oral wound healing with patent open wounds 4 weeks after tooth extraction with characteristic oral epithelial hyperplasia. γδ T cells appeared within the tooth extraction site and hyperplastic epithelium in WT ZOL mice. In ZOL-treated γδ T cell null (Tcrd(-/-) ZOL) mice, the tooth extraction open wound progressively closed; however, histological ONJ-like lesions were identified in 75 and 60% of WT ZOL and Tcrd(-/-) ZOL mice, respectively. Although the bone exposure phenotype of ONJ was predominantly observed in WT ZOL mice, Tcrd(-/-) ZOL mice developed the pustule/fistula disease phenotype. We further addressed the role of γδ T cells from human peripheral blood (h-γδ T cells). When co-cultured with ZOL-pretreated human osteoclasts in vitro, h-γδ T cells exhibited rapid expansion and robust IFN-γ secretion. When h-γδ T cells were injected into ZOL-treated immunodeficient (Rag2(-/-) ZOL) mice, the oral epithelial hyperplasia developed. However, Rag2(-/-) ZOL mice did not develop osteonecrosis. The results indicate that γδ T cells are unlikely to influence the core osteonecrosis mechanism; however, they may serve as a critical modifier contributing to the different oral mucosal disease variations of ONJ.
Keywords: ONJ; T cell; bisphosphonate; mouse; mucosal immunology; osteonecrosis; pathogenesis; wound healing; γδ T cells.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Reid I. R., Cornish J. (2012) Epidemiology and pathogenesis of osteonecrosis of the jaw. Nat. Rev. Rheumatol. 8, 90–96 - PubMed
-
- Saad F., Brown J. E., Van Poznak C., Ibrahim T., Stemmer S. M., Stopeck A. T., Diel I. J., Takahashi S., Shore N., Henry D. H., Barrios C. H., Facon T., Senecal F., Fizazi K., Zhou L., et al. (2012) Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann. Oncol. 23, 1341–1347 - PubMed
-
- Khosla S., Burr D., Cauley J., Dempster D. W., Ebeling P. R., Felsenberg D., Gagel R. F., Gilsanz V., Guise T., Koka S., McCauley L. K., McGowan J., McKee M. D., Mohla S., Pendrys D. G., et al. (2007) Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 22, 1479–1491 - PubMed
-
- Ruggiero S. L., Dodson T. B., Assael L. A., Landesberg R., Marx R. E., Mehrotra B. (2009) American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws–2009 update. J. Oral Maxillofac. Surg. 67, 2–12 - PubMed
-
- Cetiner S., Sucak G. T., Kahraman S. A., Aki S. Z., Kocakahyaoglu B., Gultekin S. E., Cetiner M., Haznedar R. (2009) Osteonecrosis of the jaw in patients with multiple myeloma treated with zoledronic acid. J. Bone Miner. Metab. 27, 435–443 - PubMed

