Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana
- PMID: 26013835
- PMCID: PMC4444972
- DOI: 10.1038/srep10533
Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana
Abstract
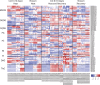
Environmental stress causes membrane damage in plants. Lipid studies are required to understand the adaptation of plants to climate change. Here, LC-MS-based lipidomic and microarray transcriptome analyses were carried out to elucidate the effect of short-term heat stress on the Arabidopsis thaliana leaf membrane. Vegetative plants were subjected to high temperatures for one day, and then grown under normal conditions. Sixty-six detected glycerolipid species were classified according to patterns of compositional change by Spearman's correlation coefficient. Triacylglycerols, 36:4- and 36:5-monogalactosyldiacylglycerol, 34:2- and 36:2-digalactosyldiacylglycerol, 34:1-, 36:1- and 36:6-phosphatidylcholine, and 34:1-phosphatidylethanolamine increased by the stress and immediately decreased during recovery. The relative amount of one triacylglycerol species (54:9) containing α-linolenic acid (18:3) increased under heat stress. These results suggest that heat stress in Arabidopsis leaves induces an increase in triacylglycerol levels, which functions as an intermediate of lipid turnover, and results in a decrease in membrane polyunsaturated fatty acids. Microarray data revealed candidate genes responsible for the observed metabolic changes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Los D. A., Mironov K. S. & Allakhverdiev S. I. Regulatory role of membrane fluidity in gene expression and physiological functions. Photosynth. Res. 116, 489–509 (2013). - PubMed
-
- Webb M. S. & Green B. R. Biochemical and biophysical properties of thylakoid acyl lipids. Biochim. Biophys. Acta 1060, 133–158 (1991).
-
- Boca S. et al. Arabidopsis lipocalins AtCHL and AtTIL have distinct but overlapping functions essential for lipid protection and seed longevity. Plant Cell Environ. 37, 368–381 (2014). - PubMed
-
- Murakami Y., Tsuyama M., Kobayashi Y., Kodama H. & Iba K. Trienoic fatty acids and plant tolerance of high temperature. Science 287, 476–479 (2000). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

