BCG+MMC trial: adding mitomycin C to BCG as adjuvant intravesical therapy for high-risk, non-muscle-invasive bladder cancer: a randomised phase III trial (ANZUP 1301)
- PMID: 26014129
- PMCID: PMC4445809
- DOI: 10.1186/s12885-015-1431-6
BCG+MMC trial: adding mitomycin C to BCG as adjuvant intravesical therapy for high-risk, non-muscle-invasive bladder cancer: a randomised phase III trial (ANZUP 1301)
Abstract
Background: Despite adequate trans-urethral resection of the bladder tumour (TURBT), non-muscle-invasive bladder cancer (NMIBC) is associated with high rates of recurrence and progression. Instillation of Bacillus Calmette-Guérin (BCG) into the urinary bladder after TURBT (adjuvant intravesical administration) reduces the risk of both recurrence and progression, and this is therefore the standard of care for high-risk tumours. However, over 30 % of people still recur or progress despite optimal delivery of BCG. Our meta-analysis suggests that outcomes might be improved further by using an adjuvant intravesical regimen that includes both mitomycin and BCG. These promising findings require corroboration in a definitive, large scale, randomised phase III trial using standard techniques for intravesical administration.
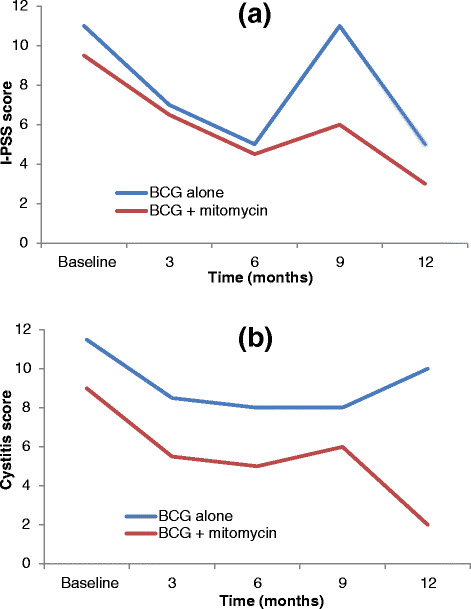
Methods and design: The BCG + MMC trial (ANZUP 1301) is an open-label, randomised, stratified, two-arm multi-centre phase III trial comparing the efficacy and safety of standard intravesical therapy (BCG alone) against experimental intravesical therapy (BCG and mitomycin) in the treatment of adults with resected, high-risk NMIBC. Participants in the control group receive standard treatment with induction (weekly BCG for six weeks) followed by maintenance (four-weekly BCG for ten months). Participants in the experimental group receive induction (BCG weeks 1, 2, 4, 5, 7, and 8; mitomycin weeks 3, 6, and 9) followed by four-weekly maintenance (mitomycin weeks 13, 17, 25, 29, 37, and 41; BCG weeks 21, 33, and 45). The trial aims to include 500 participants who will be centrally randomised to one of the two treatment groups in a 1:1 ratio stratified by T-stage, presence of CIS, and study site. The primary endpoint is disease-free survival; secondary endpoints are disease activity, time to recurrence, time to progression, safety, health-related quality of life, overall survival, feasibility, and resource use.
Trial registration: This trial is registered with the Australian New Zealand Clinical Trials Registry ( ACTRN12613000513718 ).
Figures
Similar articles
-
Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guérin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer.Eur Urol. 2016 Jun;69(6):1046-52. doi: 10.1016/j.eururo.2016.01.006. Epub 2016 Jan 20. Eur Urol. 2016. PMID: 26803476 Clinical Trial.
-
Long-term outcome of patients with frequently recurrent non-muscle-invasive bladder carcinoma treated with one perioperative plus four weekly instillations of mitomycin C followed by monthly bacillus Calmette-Guérin (BCG) or alternating BCG and interferon-α2b instillations: prospective randomised FinnBladder-4 study.Eur Urol. 2015 Oct;68(4):611-7. doi: 10.1016/j.eururo.2015.02.022. Epub 2015 Mar 5. Eur Urol. 2015. PMID: 25748117 Clinical Trial.
-
Intravesical Bacillus Calmette-Guérin versus mitomycin C for Ta and T1 bladder cancer.Cochrane Database Syst Rev. 2020 Jan 8;1(1):CD011935. doi: 10.1002/14651858.CD011935.pub2. Cochrane Database Syst Rev. 2020. PMID: 31912907 Free PMC article.
-
The effect of intravesical chemohyperthermia with mitomycin in non-muscle-invasive bladder tumour patients who cannot tolerate BCG treatment or recur after treatment and refuse cystectomy.Int Urol Nephrol. 2025 Jan;57(1):63-69. doi: 10.1007/s11255-024-04169-4. Epub 2024 Jul 31. Int Urol Nephrol. 2025. PMID: 39083172
-
Intravesical gemcitabine for non-muscle invasive bladder cancer.Cochrane Database Syst Rev. 2021 Jun 14;6(6):CD009294. doi: 10.1002/14651858.CD009294.pub3. Cochrane Database Syst Rev. 2021. PMID: 34125951 Free PMC article.
Cited by
-
New therapies in nonmuscle invasive bladder cancer treatment.Indian J Urol. 2018 Jan-Mar;34(1):11-19. doi: 10.4103/iju.IJU_296_17. Indian J Urol. 2018. PMID: 29343907 Free PMC article. Review.
-
Combination of Intravesical Bacille Calmette-Guérin and Chemotherapy vs. Bacille Calmette-Guérin Alone in Non-muscle Invasive Bladder Cancer: A Meta-Analysis.Front Oncol. 2019 Mar 1;9:121. doi: 10.3389/fonc.2019.00121. eCollection 2019. Front Oncol. 2019. PMID: 30881921 Free PMC article.
-
Adjuvant therapies for non-muscle-invasive bladder cancer: advances during BCG shortage.World J Urol. 2022 May;40(5):1111-1124. doi: 10.1007/s00345-021-03908-x. Epub 2022 Jan 27. World J Urol. 2022. PMID: 35083522 Review.
-
Impact of Effective Intravesical Therapies on Quality of Life in Patients with Non-Muscle Invasive Bladder Cancer: A Systematic Review.Int J Environ Res Public Health. 2022 Aug 30;19(17):10825. doi: 10.3390/ijerph191710825. Int J Environ Res Public Health. 2022. PMID: 36078542 Free PMC article.
-
The clinical efficacy and safety of equipment-assisted intravesical instillation of mitomycin C after transurethral resection of bladder tumour in patients with nonmuscular invasive bladder cancer: A meta-analysis.PLoS One. 2022 Oct 21;17(10):e0276453. doi: 10.1371/journal.pone.0276453. eCollection 2022. PLoS One. 2022. PMID: 36269742 Free PMC article.
References
-
- UK CR. Bladder cancer statistics. [cited 2014 16/04/2014]; Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/
-
- Babjuk M, Burger M, Zigeuner R, Shariat S, Van Rhijn B, Compérat E, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and CIS) 2013. - PubMed
-
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–75. doi: 10.1016/j.eururo.2005.12.031. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical