Late Mortality After Dexrazoxane Treatment: A Report From the Children's Oncology Group
- PMID: 26014292
- PMCID: PMC4534526
- DOI: 10.1200/JCO.2014.59.4473
Late Mortality After Dexrazoxane Treatment: A Report From the Children's Oncology Group
Abstract
Purpose: Given concerns that dexrazoxane may reduce treatment efficacy, induce second cancers, and thus compromise overall survival among children, we examined long-term overall and cause-specific mortality and disease relapse rates from three randomized clinical trials.
Patients and methods: Children's Oncology Group trials P9404 (T-cell acute lymphoblastic leukemia/lymphoma; n = 537), P9425 (intermediate/high-risk Hodgkin lymphoma; n = 216), and P9426 (low-risk Hodgkin lymphoma; n = 255) were conducted between 1996 and 2001. Each trial randomly assigned patients to doxorubicin with or without dexrazoxane. The dexrazoxane:doxorubicin dose ratio was 10:1, and the cumulative protocol-specified doxorubicin dose was 100 to 360 mg/m(2). Dexrazoxane was given as an intravenous bolus before each doxorubicin dose. Data from all three trials were linked with the National Death Index to determine overall and cause-specific mortality by dexrazoxane status.
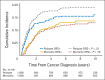
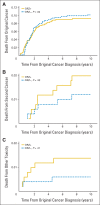
Results: Among 1,008 patients (507 received dexrazoxane) with a median follow-up of 12.6 years (range, 0 to 15.5 years), 132 died (67 received dexrazoxane). Overall mortality did not vary by dexrazoxane status (12.8% with dexrazoxane at 10 years v 12.2% without; hazard ratio [HR], 1.03; 95% CI, 0.73 to 1.45). Findings were similar when each trial was examined separately. Dexrazoxane also was not significantly associated with differential causes of death. The original cancer caused 76.5% of all deaths (HR, 0.90; 95% CI, 0.61 to 1.32) followed by second cancers (13.6% of deaths; HR, 1.24; 95% CI, 0.49 to 3.15). Specifically, dexrazoxane was not associated with deaths from acute myeloid leukemia/myelodysplasia or cardiovascular events.
Conclusion: Among pediatric patients with leukemia or lymphoma, after extended follow-up, dexrazoxane use did not seem to compromise long-term survival.
Trial registration: ClinicalTrials.gov NCT01790152.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Dexrazoxane in Children With Cancer: From Evidence to Practice.J Clin Oncol. 2015 Aug 20;33(24):2594-6. doi: 10.1200/JCO.2015.61.7928. Epub 2015 Jul 20. J Clin Oncol. 2015. PMID: 26195707 No abstract available.
References
-
- Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions—A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. - PubMed
-
- Hasinoff BB, Hellmann K, Herman EH, et al. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem. 1998;5:1–28. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

