Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment
- PMID: 26014949
- PMCID: PMC4505193
- DOI: 10.1128/AAC.00835-15
Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment
Abstract
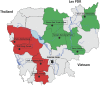
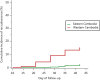
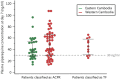
Western Cambodia is recognized as the epicenter of Plasmodium falciparum multidrug resistance. Recent reports of the efficacy of dihydroartemisinin (DHA)-piperaquine (PP), the latest of the artemisinin-based combination therapies (ACTs) recommended by the WHO, have prompted further investigations. The clinical efficacy of dihydroartemisinin-piperaquine in uncomplicated falciparum malaria was assessed in western and eastern Cambodia over 42 days. Day 7 plasma piperaquine concentrations were measured and day 0 isolates tested for in vitro susceptibilities to piperaquine and mefloquine, polymorphisms in the K13 gene, and the copy number of the Pfmdr-1 gene. A total of 425 patients were recruited in 2011 to 2013. The proportion of patients with recrudescent infections was significantly higher in western (15.4%) than in eastern (2.5%) Cambodia (P <10(-3)). Day 7 plasma PP concentrations and median 50% inhibitory concentrations (IC50) of PP were independent of treatment outcomes, in contrast to median mefloquine IC50, which were found to be lower for isolates from patients with recrudescent infections (18.7 versus 39.7 nM; P = 0.005). The most significant risk factor associated with DHA-PP treatment failure was infection by parasites carrying the K13 mutant allele (odds ratio [OR], 17.5; 95% confidence interval [CI], 1 to 308; P = 0.04). Our data show evidence of P. falciparum resistance to PP in western Cambodia, an area of widespread artemisinin resistance. New therapeutic strategies, such as the use of triple ACTs, are urgently needed and must be tested. (This study has been registered at the Australian New Zealand Clinical Trials Registry under registration no. ACTRN12614000344695.).
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- Meek SR, Doberstyn EB, Gauzere BA, Thanapanich C, Nordlander E, Phuphaisan S. 1986. Treatment of falciparum malaria with quinine and tetracycline or combined mefloquine/sulfadoxine/pyrimethamine on the Thai-Kampuchean border. Am J Trop Med Hyg 35:246–250. - PubMed
-
- Sandosham AA, Eyles DE, Montgomery R. 1964. Drug-resistance in falciparum malaria in South-East Asia. Med J Malaysia 18:172–183. - PubMed
-
- Vinayak S, Alam MT, Mixson-Hayden T, McCollum AM, Sem R, Shah NK, Lim P, Muth S, Rogers WO, Fandeur T, Barnwell JW, Escalante AA, Wongsrichanalai C, Ariey F, Meshnick SR, Udhayakumar V. 2010. Origin and evolution of sulfadoxine resistant Plasmodium falciparum. PLoS Pathog 6:e1000830. doi:10.1371/journal.ppat.1000830. - DOI - PMC - PubMed
-
- WHO. 2010. Global report on antimalarial drug efficacy and drug resistance (2000–2010). World Health Organization, Geneva, Switzerland.
-
- Denis MB, Tsuyuoka R, Lim P, Lindegårdh N, Yi P, Top SN, Socheat D, Fandeur T, Annerberg A, Christophel EM, Ringwald P. 2006. Efficacy of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Cambodia. Trop Med Int Health 11:1800–1807. doi:10.1111/j.1365-3156.2006.01739.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

