Tailoring a Pediatric Formulation of Artemether-Lumefantrine for Treatment of Plasmodium falciparum Malaria
- PMID: 26014953
- PMCID: PMC4505267
- DOI: 10.1128/AAC.00014-15
Tailoring a Pediatric Formulation of Artemether-Lumefantrine for Treatment of Plasmodium falciparum Malaria
Abstract
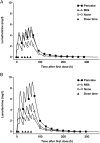
Specially created pediatric formulations have the potential to improve the acceptability, effectiveness, and accuracy of dosing of artemisinin-based combination therapy (ACT) in young children, a patient group that is inherently vulnerable to malaria. Artemether-lumefantrine (AL) Dispersible is a pediatric formulation of AL that is specifically tailored for the treatment of children with uncomplicated Plasmodium falciparum malaria, offering benefits relating to efficacy, convenience and acceptance, accuracy of dosing, safety, sterility, stability, and a pharmacokinetic profile and bioequivalence similar to those of crushed and intact AL tablets. However, despite being the first pediatric antimalarial to meet World Health Organization (WHO) specifications for use in infants and children who are ≥5 kg in body weight and its inclusion in WHO Guidelines, there are few publications that focus on AL Dispersible. Based on a systematic review of the recent literature, this paper provides a comprehensive overview of the clinical experience with AL Dispersible to date. A randomized, phase 3 study that compared the efficacy and safety of AL Dispersible to those of crushed AL tablets in 899 African children reported high PCR-corrected cure rates at day 28 (97.8% and 98.5% for AL Dispersible and crushed tablets, respectively), and the results of several subanalyses of these data indicate that this activity is observed regardless of patient weight, food intake, and maximum plasma concentrations of artemether or its active metabolite, dihydroartemisinin. These and other clinical data support the continued use of pediatric antimalarial formulations in all children <5 years of age with uncomplicated malaria when accompanied by continued monitoring for the emergence of resistance.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- WHO. 2014. World malaria report 2014. World Health Organization, Geneva, Switzerland.
-
- WHO. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

