Biomaterial-mediated strategies targeting vascularization for bone repair
- PMID: 26014967
- PMCID: PMC4662653
- DOI: 10.1007/s13346-015-0236-0
Biomaterial-mediated strategies targeting vascularization for bone repair
Abstract
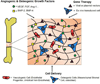
Repair of non-healing bone defects through tissue engineering strategies remains a challenging feat in the clinic due to the aversive microenvironment surrounding the injured tissue. The vascular damage that occurs following a bone injury causes extreme ischemia and a loss of circulating cells that contribute to regeneration. Tissue-engineered constructs aimed at regenerating the injured bone suffer from complications based on the slow progression of endogenous vascular repair and often fail at bridging the bone defect. To that end, various strategies have been explored to increase blood vessel regeneration within defects to facilitate both tissue-engineered and natural repair processes. Developments that induce robust vascularization will need to consolidate various parameters including optimization of embedded therapeutics, scaffold characteristics, and successful integration between the construct and the biological tissue. This review provides an overview of current strategies as well as new developments in engineering biomaterials to induce reparation of a functional vascular supply in the context of bone repair.
Keywords: Bone regeneration; Cell therapy; Growth factor; Tissue engineering; Vascularization.
Conflict of interest statement
The authors report no conflict of interest
Figures

References
-
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4–5):300–311. - PubMed
-
- Santos MI, Reis RL. Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci. 2010;10(1):12–27. - PubMed
-
- Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

