YAP is a critical oncogene in human cholangiocarcinoma
- PMID: 26015398
- PMCID: PMC4627302
- DOI: 10.18632/oncotarget.4043
YAP is a critical oncogene in human cholangiocarcinoma
Abstract
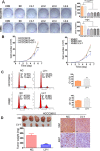
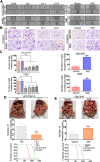
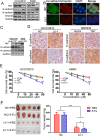
Yes-associated protein (YAP), a transcriptional co-activator, has important regulatory roles in cell signaling and is dysregulated in a number of cancers. However, the role of YAP in cholangiocarcinoma (CCA) progression remains unclear. Here, we demonstrated that YAP was overexpressed in CCA cells and human specimens. High levels of nuclear YAP (nYAP) correlated with histological differentiation, TNM stage, metastasis and poor prognosis in CCA. Silencing YAP increased tumor sensitivity to chemotherapy and inhibited CCA tumorigenesis and metastasis both in vivo and in vitro. YAP overexpression in vivo and in vitro promoted CCA tumorigenesis and metastasis. Additionally, we found that YAP induced epithelial-mesenchymal transition (EMT) and formed a regulatory circuit with miR-29c, IGF1, AKT and gankyrin to promote the progression of CCA. Results of CCA tissue microarray showed positive correlations between nYAP and gankyrin or p-AKT expression. Combination of nYAP and gankyrin or p-AKT exhibited improved prognostic accuracy for CCA patients. In conclusion, YAP promotes carcinogenesis and metastasis by up-regulating gankyrin through activation of the AKT pathway.
Keywords: AKT; YAP; cholangiocarcinoma; gankyrin; tumorigenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303–1314. - PubMed
-
- Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. Journal of hepatology. 2002;37:806–813. - PubMed
-
- Lau SH, Lau WY. Current therapy of hilar cholangiocarcinoma. Hepatobiliary & pancreatic diseases international : HBPD INT. 2012;11:12–17. - PubMed
-
- Romiti A, D'Antonio C, Zullo A, Sarcina I, Di Rocco R, Barucca V, Durante V, Marchetti P. Chemotherapy for the biliary tract cancers: moving toward improved survival time. Journal of gastrointestinal cancer. 2012;43:396–404. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

