Micropatterned macrophage analysis reveals global cytoskeleton constraints induced by Bacillus anthracis edema toxin
- PMID: 26015478
- PMCID: PMC4496603
- DOI: 10.1128/IAI.00479-15
Micropatterned macrophage analysis reveals global cytoskeleton constraints induced by Bacillus anthracis edema toxin
Abstract
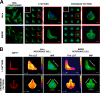
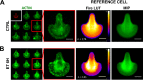
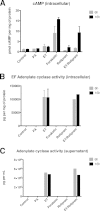
Bacillus anthracis secretes the edema toxin (ET) that disrupts the cellular physiology of endothelial and immune cells, ultimately affecting the adherens junction integrity of blood vessels that in turn leads to edema. The effects of ET on the cytoskeleton, which is critical in cell physiology, have not been described thus far on macrophages. In this study, we have developed different adhesive micropatterned surfaces (L and crossbow) to control the shape of bone marrow-derived macrophages (BMDMs) and primary peritoneal macrophages. We found that macrophage F-actin cytoskeleton adopts a specific polar organization slightly different from classical human HeLa cells on the micropatterns. Moreover, ET induced a major quantitative reorganization of F-actin within 16 h with a collapse at the nonadhesive side of BMDMs along the nucleus. There was an increase in size and deformation into a kidney-like shape, followed by a decrease in size that correlates with a global cellular collapse. The collapse of F-actin was correlated with a release of focal adhesion on the patterns and decreased cell size. Finally, the cell nucleus was affected by actin reorganization. By using this technology, we could describe many previously unknown macrophage cellular dysfunctions induced by ET. This novel tool could be used to analyze more broadly the effects of toxins and other virulence factors that target the cytoskeleton.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
The effect of deletion of the edema factor on Bacillus anthracis pathogenicity in guinea pigs and rabbits.Microb Pathog. 2012 Jan;52(1):55-60. doi: 10.1016/j.micpath.2011.10.002. Epub 2011 Oct 17. Microb Pathog. 2012. PMID: 22020310
-
cAMP signaling by anthrax edema toxin induces transendothelial cell tunnels, which are resealed by MIM via Arp2/3-driven actin polymerization.Cell Host Microbe. 2011 Nov 17;10(5):464-74. doi: 10.1016/j.chom.2011.09.014. Cell Host Microbe. 2011. PMID: 22100162
-
c-di-AMP accumulation impairs toxin expression of Bacillus anthracis by down-regulating potassium importers.Microbiol Spectr. 2024 Aug 6;12(8):e0378623. doi: 10.1128/spectrum.03786-23. Epub 2024 Jun 20. Microbiol Spectr. 2024. PMID: 38899864 Free PMC article.
-
Cytoskeleton as an emerging target of anthrax toxins.Toxins (Basel). 2012 Feb;4(2):83-97. doi: 10.3390/toxins4020083. Epub 2012 Feb 6. Toxins (Basel). 2012. PMID: 22474568 Free PMC article. Review.
-
New aspects of the infection mechanisms of Bacillus anthracis.Ann Agric Environ Med. 2012;19(4):613-8. Ann Agric Environ Med. 2012. PMID: 23311776 Review.
Cited by
-
Calcium Signaling in Pancreatic Immune Cells In situ.Function (Oxf). 2020 Oct 13;2(1):zqaa026. doi: 10.1093/function/zqaa026. eCollection 2021. Function (Oxf). 2020. PMID: 35330972 Free PMC article.
-
Bacillus anthracis, "la maladie du charbon", Toxins, and Institut Pasteur.Toxins (Basel). 2024 Jan 26;16(2):66. doi: 10.3390/toxins16020066. Toxins (Basel). 2024. PMID: 38393144 Free PMC article. Review.
-
Crossing of the epithelial barriers by Bacillus anthracis: the Known and the Unknown.Front Microbiol. 2015 Oct 9;6:1122. doi: 10.3389/fmicb.2015.01122. eCollection 2015. Front Microbiol. 2015. PMID: 26500645 Free PMC article. Review.
-
The Saccharomyces boulardii CNCM I-745 strain shows protective effects against the B. anthracis LT toxin.Toxins (Basel). 2015 Oct 30;7(11):4455-67. doi: 10.3390/toxins7114455. Toxins (Basel). 2015. PMID: 26529015 Free PMC article.
-
Capturing extracellular matrix properties in vitro: Microengineering materials to decipher cell and tissue level processes.Exp Biol Med (Maywood). 2016 May;241(9):930-8. doi: 10.1177/1535370216644532. Epub 2016 Apr 12. Exp Biol Med (Maywood). 2016. PMID: 27075930 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

