Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium
- PMID: 26015562
- PMCID: PMC4466733
- DOI: 10.1073/pnas.1504177112
Neural Hedgehog signaling maintains stem cell renewal in the sensory touch dome epithelium
Abstract
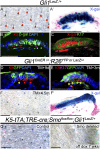
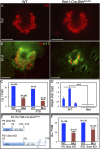
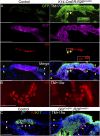
The touch dome is a highly patterned mechanosensory structure in the epidermis composed of specialized keratinocytes in juxtaposition with innervated Merkel cells. The touch dome epithelium is maintained by tissue-specific stem cells, but the signals that regulate the touch dome are not known. We identify touch dome stem cells that are unique among epidermal cells in their activated Hedgehog signaling and ability to maintain the touch dome as a distinct lineage compartment. Skin denervation reveals that renewal of touch dome stem cells requires a perineural microenvironment, and deleting Sonic hedgehog (Shh) in neurons or Smoothened in the epidermis demonstrates that Shh is an essential niche factor that maintains touch dome stem cells. Up-regulation of Hedgehog signaling results in neoplastic expansion of touch dome keratinocytes but no Merkel cell neoplasia. These findings demonstrate that nerve-derived Shh is a critical regulator of lineage-specific stem cells that maintain specialized sensory compartments in the epidermis.
Keywords: Hedgehog; Merkel cell; perineural niche; stem cell; touch dome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Morgan BA. 2014. The dermal papilla: An instructive niche for epithelial stem and progenitor cells in development and regeneration of the hair follicle. Cold Spring Harb Perspect Med 4(7):a015180.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

