Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans
- PMID: 26015572
- PMCID: PMC4466753
- DOI: 10.1073/pnas.1508224112
Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans
Abstract
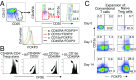
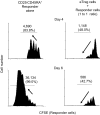
CD4(+) regulatory T (Treg) cells expressing CD25 and the transcription factor forkhead box P3 (FOXP3) are indispensable for immunological self-tolerance and homeostasis. FOXP3(+)CD25(+)CD4(+) T cells in humans, however, are heterogeneous in function and differentiation status, including suppressive or nonsuppressive cells as well as resting or activated Treg cells. We have searched for cell surface markers specific for suppression-competent Treg cells by using a panel of currently available monoclonal antibodies reactive with human T cells. We found that CD15s (sialyl Lewis x) was highly specific for activated, terminally differentiated, and most suppressive FOXP3(high) effector Treg (eTreg) cells and able to differentiate them in various clinical settings from nonsuppressive FOXP3(+) T cells secreting inflammatory cytokines. For example, CD15s(+)FOXP3(+) eTreg cells were increased in sarcoidosis, whereas it was nonsuppressive CD15s(-)FOXP3(+) T cells that were expanded in lupus flares. FOXP3(+) cells induced from conventional CD4(+) T cells by T-cell receptor stimulation hardly expressed CD15s. CD15s(+)CD4(+) T-cell depletion was sufficient to evoke and enhance in vitro immune responses against tumor or viral antigens. Collectively, we have identified CD15s as a biomarker instrumental in both phenotypic and functional analysis of FOXP3(+)CD4(+) T-cell subpopulations in health and disease. It allows specific targeting of eTreg cells, rather than whole FOXP3(+)CD4(+) T cells, in controlling immune responses.
Keywords: CD15s; FOXP3; autoimmunity; regulatory T cells; tumor immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


















References
-
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. - PubMed
-
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490–500. - PubMed
-
- Allan SE, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19(4):345–354. - PubMed
-
- Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

