Mutation signatures implicate aristolochic acid in bladder cancer development
- PMID: 26015808
- PMCID: PMC4443665
- DOI: 10.1186/s13073-015-0161-3
Mutation signatures implicate aristolochic acid in bladder cancer development
Abstract
Background: Aristolochic acid (AA) is a natural compound found in many plants of the Aristolochia genus, and these plants are widely used in traditional medicines for numerous conditions and for weight loss. Previous work has connected AA-mutagenesis to upper-tract urothelial cell carcinomas and hepatocellular carcinomas. We hypothesize that AA may also contribute to bladder cancer.
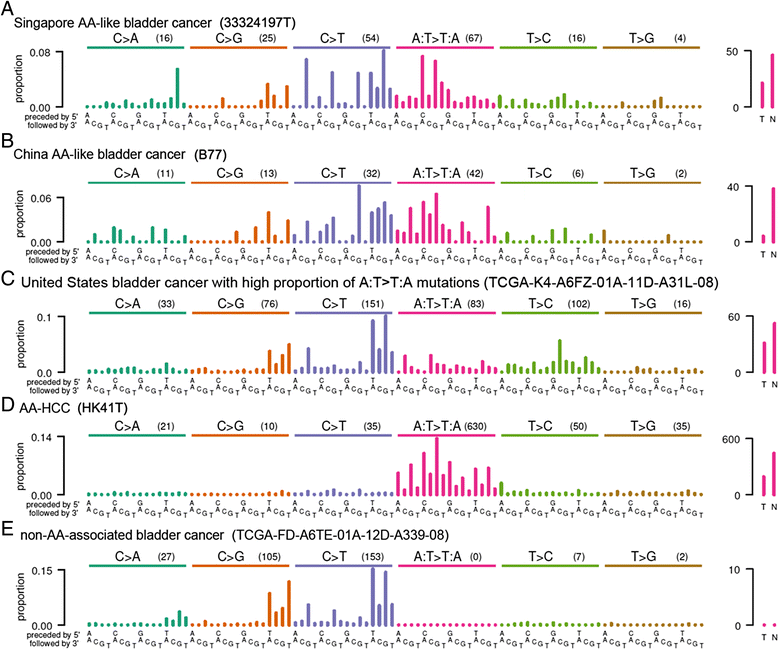
Methods: Here, we investigated the involvement of AA-mutagenesis in bladder cancer by sequencing bladder tumor genomes from two patients with known exposure to AA. After detecting strong mutational signatures of AA exposure in these tumors, we exome-sequenced and analyzed an additional 11 bladder tumors and analyzed publicly available somatic mutation data from a further 336 bladder tumors.
Results: The somatic mutations in the bladder tumors from the two patients with known AA exposure showed overwhelming AA signatures. We also detected evidence of AA exposure in 1 out of 11 bladder tumors from Singapore and in 3 out of 99 bladder tumors from China. In addition, 1 out of 194 bladder tumors from North America showed a pattern of mutations that might have resulted from exposure to an unknown mutagen with a heretofore undescribed pattern of A > T mutations. Besides the signature of AA exposure, the bladder tumors also showed the CpG > TpG and activated-APOBEC signatures, which have been previously reported in bladder cancer.
Conclusions: This study demonstrates the utility of inferring mutagenic exposures from somatic mutation spectra. Moreover, AA exposure in bladder cancer appears to be more pervasive in the East, where traditional herbal medicine is more widely used. More broadly, our results suggest that AA exposure is more extensive than previously thought both in terms of populations at risk and in terms of types of cancers involved. This appears to be an important public health issue that should be addressed by further investigation and by primary prevention through regulation and education. In addition to opportunities for primary prevention, knowledge of AA exposure would provide opportunities for secondary prevention in the form of intensified screening of patients with known or suspected AA exposure.
Figures


References
-
- Howe G, Burch J, Miller A, Cook G, Esteve J, Morrison B, et al. Tobacco use, occupation, coffee, various nutrients, and bladder cancer. J Natl Cancer Inst. 1980;64:701–13. - PubMed
-
- Bates MN, Smith AH, Hopenhayn-Rich C. Arsenic ingestion and internal cancers: a review. Am J Epidemiol. 1992;135:462–76. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

