Role of endothelin-1 antagonist; bosentan, against cisplatin-induced nephrotoxicity in male and female rats
- PMID: 26015909
- PMCID: PMC4434484
- DOI: 10.4103/2277-9175.156642
Role of endothelin-1 antagonist; bosentan, against cisplatin-induced nephrotoxicity in male and female rats
Abstract
Background: Cisplatin (CP) is a chemotherapy drug, with the major side effect of nephrotoxicity. The level of endothelin-1 (ET-1) increases during nephrotoxicity, which is accompanied with vasoconstrictive properties. Bosentan (BOS) is a nonselective ET-1 receptor antagonist, having vasodilatory and anti-hypertension effects. The purpose of this study was to investigate the renoprotective effect of BOS against CP-induced nephrotoxicity in male and female rats.
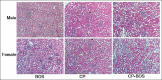
Materials and methods: Male and female rats were divided into six groups; groups 1-3 and 4-6 were male and female rats, respectively. Animals in groups 1 and 4 were considered as negative control and groups 2 and 5 considered as positive control groups received BOS (30 mg/kg/day) alone and CP (2.5 mg/kg/day) alone, respectively, for 1-week. The animals in groups 3 and 6 were treated with both CP and BOS. Finally, serum parameters were measured, and the kidney tissue was subjected to staining to evaluate tissue damage.
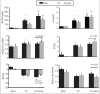
Results: The serum levels of blood urea nitrogen and creatinine, kidney tissue damage score and kidney weight elevated, and body weight significantly decreased in both CP alone and in CP plus BOS-treated groups when compared with the control groups (P < 0.05), while BOS did not ameliorate these parameters neither in males nor in females. No significant differences were observed in serum levels of nitrite and malondialdehyde between the groups, but kidney tissue level of nitrite decreased significantly in CP alone and CP plus BOS-treated groups (P < 0.05).
Conclusion: Renoprotective effect of BOS, as ET-1 blocker, was not observed against CP-induced nephrotoxicity neither in male nor in female rats. This is while BOS promoted the severity of injuries in females.
Keywords: Bosentan; cisplatin; gender; nephrotoxicity; rat.
Conflict of interest statement
Figures
Similar articles
-
Sex Difference in Cisplatin-Induced Nephrotoxicity: Laboratory and Clinical Findings.J Toxicol. 2022 Oct 10;2022:3507721. doi: 10.1155/2022/3507721. eCollection 2022. J Toxicol. 2022. PMID: 36263084 Free PMC article. Review.
-
Role of endothelin receptor antagonist; bosentan in cisplatin-induced nephrotoxicity in ovariectomized estradiol treated rats.J Nephropathol. 2015 Oct;4(4):134-40. doi: 10.12860/jnp.2015.25. Epub 2015 Oct 1. J Nephropathol. 2015. PMID: 26457261 Free PMC article.
-
The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences.Ren Fail. 2012;34(8):1046-51. doi: 10.3109/0886022X.2012.700886. Epub 2012 Jul 11. Ren Fail. 2012. PMID: 22780575
-
Effect of enalapril in cisplatin-induced nephrotoxicity in rats; gender-related difference.Adv Biomed Res. 2016 Jan 29;5:14. doi: 10.4103/2277-9175.175253. eCollection 2016. Adv Biomed Res. 2016. PMID: 26962516 Free PMC article.
-
Nonpreventive Role of Aerobic Exercise Against Cisplatin-induced Nephrotoxicity in Female Rats.Int J Prev Med. 2015 Jul 8;6:58. doi: 10.4103/2008-7802.160333. eCollection 2015. Int J Prev Med. 2015. PMID: 26288702 Free PMC article.
Cited by
-
Endothelin-1 and the kidney: new perspectives and recent findings.Curr Opin Nephrol Hypertens. 2016 Jan;25(1):35-41. doi: 10.1097/MNH.0000000000000185. Curr Opin Nephrol Hypertens. 2016. PMID: 26625864 Free PMC article. Review.
-
Age- and Gender-Related Differences in Renal Vascular Responses to Angiotensin II in Rats: The Role of the Mas Receptor.J Aging Res. 2023 Aug 16;2023:3560468. doi: 10.1155/2023/3560468. eCollection 2023. J Aging Res. 2023. PMID: 37622033 Free PMC article.
-
The Effect of Angiotensin II Type 1 Receptor Antagonist on Age-Related Differences in Renal Vascular Responses to Angiotensin II in Male and Female Rats.Adv Biomed Res. 2024 Aug 26;13:71. doi: 10.4103/abr.abr_387_23. eCollection 2024. Adv Biomed Res. 2024. PMID: 39434945 Free PMC article.
-
Sex Difference in Cisplatin-Induced Nephrotoxicity: Laboratory and Clinical Findings.J Toxicol. 2022 Oct 10;2022:3507721. doi: 10.1155/2022/3507721. eCollection 2022. J Toxicol. 2022. PMID: 36263084 Free PMC article. Review.
-
Gender and Sex-Related Differences in Normal Tissue Effects Induced by Platinum Compounds.Pharmaceuticals (Basel). 2022 Feb 20;15(2):255. doi: 10.3390/ph15020255. Pharmaceuticals (Basel). 2022. PMID: 35215367 Free PMC article. Review.
References
-
- Hartmann JT, Fels LM, Knop S, Stolt H, Kanz L, Bokemeyer C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide–based combination chemotherapy with or without amifostine in patients with solid tumors. Invest New Drugs. 2000;18:281–9. - PubMed
-
- Hartmann JT, Knop S, Fels LM, van Vangerow A, Stolte H, Kanz L, et al. The use of reduced doses of amifostine to ameliorate nephrotoxicity of cisplatin/ifosfamide-based chemotherapy in patients with solid tumors. Anticancer Drugs. 2000;11:1–6. - PubMed
-
- Blakley BW, Gupta AK, Myers SF, Schwan S. Risk factors for ototoxicity due to cisplatin. Arch Otolaryngol Head Neck Surg. 1994;120:541–6. - PubMed
-
- Liu J, Liu Y, Habeebu SS, Klaassen CD. Metallothionein (MT)-null mice are sensitive to cisplatin-induced hepatotoxicity. Toxicol Appl Pharmacol. 1998;149:24–31. - PubMed
-
- Nematbakhsh M, Ashrafi F, Pezeshki Z, Fatahi Z, Kianpoor F, Sanei MH, et al. A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: Which one is the first observation as side effect of Cisplatin-induced toxicity in animal model? J Nephropathol. 2012;1:190–3. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

