AAV8-mediated Sirt1 gene transfer to the liver prevents high carbohydrate diet-induced nonalcoholic fatty liver disease
- PMID: 26015978
- PMCID: PMC4362360
- DOI: 10.1038/mtm.2014.39
AAV8-mediated Sirt1 gene transfer to the liver prevents high carbohydrate diet-induced nonalcoholic fatty liver disease
Abstract
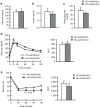
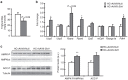
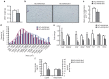
Nonalcoholic fatty liver disease (NAFLD) is the most common hepatic disease worldwide, and evidence suggests that it promotes insulin resistance and type 2 diabetes. Caloric restriction (CR) is the only available strategy for NAFLD treatment. The protein deacetylase Sirtuin1 (SIRT1), which is activated by CR, increases catabolic metabolism and decreases lipogenesis and inflammation, both involved in the development of NAFLD. Here we show that adeno-associated viral vectors of serotype 8 (AAV8)-mediated liver-specific Sirt1 gene transfer prevents the development of NAFLD induced by a high carbohydrate (HC) diet. Long-term hepatic SIRT1 overexpression led to upregulation of key hepatic genes involved in β-oxidation, prevented HC diet-induced lipid accumulation and reduced liver inflammation. AAV8-Sirt1-treated mice showed improved insulin sensitivity, increased oxidative capacity in skeletal muscle and reduced white adipose tissue inflammation. Moreover, HC feeding induced leptin resistance, which was also attenuated in AAV8-Sirt1-treated mice. Therefore, AAV-mediated gene transfer to overexpress SIRT1 specifically in the liver may represent a new gene therapy strategy to counteract NAFLD and related diseases such as type 2 diabetes.
Figures




References
-
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2012;8:228–236. - PubMed
-
- Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7:251–264. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

