Optimization of a gene electrotransfer procedure for efficient intradermal immunization with an hTERT-based DNA vaccine in mice
- PMID: 26015983
- PMCID: PMC4362362
- DOI: 10.1038/mtm.2014.45
Optimization of a gene electrotransfer procedure for efficient intradermal immunization with an hTERT-based DNA vaccine in mice
Abstract
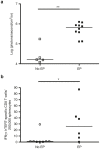
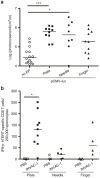
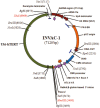
DNA vaccination consists in administering an antigen-encoding plasmid in order to trigger a specific immune response. This specific vaccine strategy is of particular interest to fight against various infectious diseases and cancer. Gene electrotransfer is the most efficient and safest non-viral gene transfer procedure and specific electrical parameters have been developed for several target tissues. Here, a gene electrotransfer protocol into the skin has been optimized in mice for efficient intradermal immunization against the well-known telomerase tumor antigen. First, the luciferase reporter gene was used to evaluate gene electrotransfer efficiency into the skin as a function of the electrical parameters and electrodes, either non-invasive or invasive. In a second time, these parameters were tested for their potency to generate specific cellular CD8 immune responses against telomerase epitopes. These CD8 T-cells were fully functional as they secreted IFNγ and were endowed with specific cytotoxic activity towards target cells. This simple and optimized procedure for efficient gene electrotransfer into the skin using the telomerase antigen is to be used in cancer patients for the phase 1 clinical evaluation of a therapeutic cancer DNA vaccine called INVAC-1.
Figures





Similar articles
-
Anticancer DNA vaccine based on human telomerase reverse transcriptase generates a strong and specific T cell immune response.Oncoimmunology. 2015 Nov 5;5(3):e1083670. doi: 10.1080/2162402X.2015.1083670. eCollection 2016 Mar. Oncoimmunology. 2015. PMID: 27141336 Free PMC article.
-
Optimisation of intradermal DNA electrotransfer for immunisation.J Control Release. 2007 Dec 4;124(1-2):81-7. doi: 10.1016/j.jconrel.2007.08.010. Epub 2007 Aug 19. J Control Release. 2007. PMID: 17854939
-
A First-in-Human Phase I Study of INVAC-1, an Optimized Human Telomerase DNA Vaccine in Patients with Advanced Solid Tumors.Clin Cancer Res. 2020 Feb 1;26(3):588-597. doi: 10.1158/1078-0432.CCR-19-1614. Epub 2019 Sep 26. Clin Cancer Res. 2020. PMID: 31558479
-
Genetic immunization with plasmid DNA mediated by electrotransfer.Hum Gene Ther. 2011 Jul;22(7):789-98. doi: 10.1089/hum.2011.092. Hum Gene Ther. 2011. PMID: 21631165 Review.
-
Gene electrotransfer to skin; review of existing literature and clinical perspectives.Curr Gene Ther. 2010 Aug;10(4):287-99. doi: 10.2174/156652310791823443. Curr Gene Ther. 2010. PMID: 20557284 Review.
Cited by
-
Plasmid DNA Delivery into the Skin via Electroporation with a Depot-Type Electrode.Pharmaceutics. 2024 Sep 18;16(9):1219. doi: 10.3390/pharmaceutics16091219. Pharmaceutics. 2024. PMID: 39339255 Free PMC article.
-
Exploring the Applicability of Nano-Poration for Remote Control in Smart Drug Delivery Systems.J Membr Biol. 2017 Feb;250(1):31-40. doi: 10.1007/s00232-016-9922-1. Epub 2016 Aug 25. J Membr Biol. 2017. PMID: 27561639
-
A DNA telomerase vaccine for canine cancer immunotherapy.Oncotarget. 2019 May 21;10(36):3361-3372. doi: 10.18632/oncotarget.26927. eCollection 2019 May 21. Oncotarget. 2019. PMID: 31164958 Free PMC article.
-
Immunogenic Effects and Clinical Applications of Electroporation-Based Treatments.Vaccines (Basel). 2023 Dec 30;12(1):42. doi: 10.3390/vaccines12010042. Vaccines (Basel). 2023. PMID: 38250855 Free PMC article.
-
Perspectives on Transdermal Electroporation.Pharmaceutics. 2016 Mar 17;8(1):9. doi: 10.3390/pharmaceutics8010009. Pharmaceutics. 2016. PMID: 26999191 Free PMC article. Review.
References
-
- André FM, Gehl J, Sersa G, Préat V, Hojman P, Eriksen J. Efficiency of high- and low-voltage pulse combinations for gene electrotransfer in muscle, liver, tumor, and skin. Hum Gene Ther. 2008;19:1261–1271. - PubMed
-
- Gothelf A, Gehl J. Gene electrotransfer to skin; review of existing literature and clinical perspectives. Curr Gene Ther. 2010;10:287–299. - PubMed
-
- Rochard A, Scherman D, Bigey P. Genetic immunization with plasmid DNA mediated by electrotransfer. Hum Gene Ther. 2011;22:789–798. - PubMed
-
- Andre FM, Mir LM. Nucleic acids electrotransfer in vivo: mechanisms and practical aspects. Curr Gene Ther. 2010;10:267–280. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

