Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study
- PMID: 26016488
- PMCID: PMC4445790
- DOI: 10.1136/bmj.h2445
Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study
Abstract
Objective: To examine whether deviation from the standard intention to treat analysis has an influence on treatment effect estimates of randomised trials.
Design: Meta-epidemiological study.
Data sources: Medline, via PubMed, searched between 2006 and 2010; 43 systematic reviews of interventions and 310 randomised trials were included.
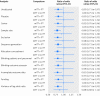
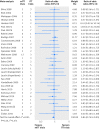
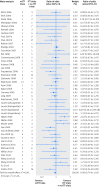
Eligibility criteria for selecting studies: From each year searched, random selection of 5% of intervention reviews with a meta-analysis that included at least one trial that deviated from the standard intention to treat approach. Basic characteristics of the systematic reviews and randomised trials were extracted. Information on the reporting of intention to treat analysis, outcome data, risk of bias items, post-randomisation exclusions, and funding were extracted from each trial. Trials were classified as: ITT (reporting the standard intention to treat approach), mITT (reporting a deviation from the standard approach), and no ITT (reporting no approach). Within each meta-analysis, treatment effects were compared between mITT and ITT trials, and between mITT and no ITT trials. The ratio of odds ratios was calculated (value <1 indicated larger treatment effects in mITT trials than in other trial categories).
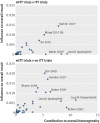
Results: 50 meta-analyses and 322 comparisons of randomised trials (from 84 ITT trials, 118 mITT trials, and 108 no ITT trials; 12 trials contributed twice to the analysis) were examined. Compared with ITT trials, mITT trials showed a larger intervention effect (pooled ratio of odds ratios 0.83 (95% confidence interval 0.71 to 0.96), P=0.01; between meta-analyses variance τ(2)=0.13). Adjustments for sample size, type of centre, funding, items of risk of bias, post-randomisation exclusions, and variance of log odds ratio yielded consistent results (0.80 (0.69 to 0.94), P=0.005; τ(2)=0.08). After exclusion of five influential studies, results remained consistent (0.85 (0.75 to 0.98); τ(2)=0.08). The comparison between mITT trials and no ITT trials showed no statistical difference between the two groups (adjusted ratio of odds ratios 0.92 (0.70 to 1.23); τ(2)=0.57).
Conclusions: Trials that deviated from the intention to treat analysis showed larger intervention effects than trials that reported the standard approach. Where an intention to treat analysis is impossible to perform, authors should clearly report who is included in the analysis and attempt to perform multiple imputations.
© Abraha et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

References
-
- Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clin Trials 2004;1:368-76. - PubMed
-
- Newell DJ. Intention-to-treat analysis: implications for quantitative and qualitative research. Int J Epidemiol 1992;21:837-41. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
