Aggregated clumps of lithistid sponges: a singular, reef-like bathyal habitat with relevant paleontological connections
- PMID: 26016786
- PMCID: PMC4446211
- DOI: 10.1371/journal.pone.0125378
Aggregated clumps of lithistid sponges: a singular, reef-like bathyal habitat with relevant paleontological connections
Abstract
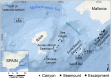
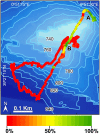
The advent of deep-sea exploration using video cameras has uncovered extensive sponge aggregations in virtually all oceans. Yet, a distinct type is herein reported from the Mediterranean: a monospecific reef-like formation built by the lithistid demosponge Leiodermatium pfeifferae. Erect, plate-like individuals (up to 80 cm) form bulky clumps, making up to 1.8 m high mounds (1.14 m on average) on the bottom, at a 760 m-deep seamount named SSS. The siliceous skeletal frameworks of the lithistids persist after sponge death, serving as a complex 3D substratum where new lithistids recruit, along with a varied fauna of other sessile and vagile organisms. The intricate aggregation of lithistid mounds functions as a "reef" formation, architecturally different from the archetypal "demosponge gardens" with disaggregating siliceous skeletons. Leiodermatium pfeifferae also occurred at two additional, close seamounts (EBJ and EBS), but, unlike at SSS, the isolated individuals never formed accretive clumps. The general oceanographic variables (temperature, salinity, dissolved nutrients, chlorophyll, and oxygen) revealed only minimal between-seamount differences, which cannot explain why sponge abundance at SSS is about two orders of magnitude higher than at EBJ or EBS. Large areas of the dense SSS aggregation were damaged, with detached and broken sponges and a few tangled fishing lines. Satellite vessel monitoring revealed low fishing activity around these seamounts. In contrast, international plans for gas and oil extraction at those locations raise serious concerns over the need for protecting urgently this unique, vulnerable habitat to avoid further alteration. Modern lithistids are a relict fauna from Jurassic and Cretaceous reefs and the roots of the very genus Leiodermatium can be traced back to those fossil formations. Therefore, understanding the causes behind the discovered lithistid aggregation is critical not only to its preservation, but also to elucidate how the extraordinary Mesozoic lithistid formations developed and functioned.
Conflict of interest statement
Figures





Similar articles
-
Rock sponges (lithistid Demospongiae) of the Northeast Atlantic seamounts, with description of ten new species.PeerJ. 2020 Apr 7;8:e8703. doi: 10.7717/peerj.8703. eCollection 2020. PeerJ. 2020. PMID: 32292645 Free PMC article.
-
Status of the glass sponge reefs in the Georgia Basin.Mar Environ Res. 2008 Dec;66 Suppl:S80-6. doi: 10.1016/j.marenvres.2008.09.002. Epub 2008 Sep 21. Mar Environ Res. 2008. PMID: 18954900
-
Systematics of 'lithistid' tetractinellid demosponges from the Tropical Western Atlantic-implications for phylodiversity and bathymetric distribution.PeerJ. 2021 Apr 2;9:e10775. doi: 10.7717/peerj.10775. eCollection 2021. PeerJ. 2021. PMID: 33859870 Free PMC article.
-
Potential Impacts of Offshore Oil and Gas Activities on Deep-Sea Sponges and the Habitats They Form.Adv Mar Biol. 2018;79:33-60. doi: 10.1016/bs.amb.2018.01.001. Epub 2018 Mar 9. Adv Mar Biol. 2018. PMID: 30012276 Review.
-
The role of sponges in the Mesoamerican Barrier-Reef Ecosystem, Belize.Adv Mar Biol. 2012;61:211-71. doi: 10.1016/B978-0-12-387787-1.00002-7. Adv Mar Biol. 2012. PMID: 22560779 Review.
Cited by
-
Rock sponges (lithistid Demospongiae) of the Northeast Atlantic seamounts, with description of ten new species.PeerJ. 2020 Apr 7;8:e8703. doi: 10.7717/peerj.8703. eCollection 2020. PeerJ. 2020. PMID: 32292645 Free PMC article.
-
Diversity of a bacterial community associated with Cliona lobata Hancock and Gelliodes pumila (Lendenfeld, 1887) sponges on the South-East coast of India.Sci Rep. 2020 Jul 14;10(1):11558. doi: 10.1038/s41598-020-67717-9. Sci Rep. 2020. PMID: 32665602 Free PMC article.
-
Trophic ecology of glass sponge reefs in the Strait of Georgia, British Columbia.Sci Rep. 2018 Jan 15;8(1):756. doi: 10.1038/s41598-017-19107-x. Sci Rep. 2018. PMID: 29335445 Free PMC article.
-
From caves to seamounts: the hidden diversity of tetractinellid sponges from the Balearic Islands, with the description of eight new species.PeerJ. 2024 Mar 4;12:e16584. doi: 10.7717/peerj.16584. eCollection 2024. PeerJ. 2024. PMID: 39670108 Free PMC article.
-
Sponges of Western Mediterranean seamounts: new genera, new species and new records.PeerJ. 2021 Aug 26;9:e11879. doi: 10.7717/peerj.11879. eCollection 2021. PeerJ. 2021. PMID: 34527436 Free PMC article.
References
-
- Reiswig HM. Water transport, respiration and energetics of three tropical marine sponges. J Exp Mar Biol Ecol. 1974;14: 231–249.
-
- Pile AJ, Young CM. The natural diet of a hexactinellid sponge: Benthic-pelagic coupling in a deep-sea microbial food web. Deep-Sea Res. 2006;I 53: 1148–1156.
-
- Bell JJ. The functional roles of marine sponges. Estuar Coast Shelf Sci. 2008;79: 341–353.
-
- Buhl-Mortensen L, Vanreusel A, Gooday AJ, Levin LA, Priede IG, Buhl-Mortensen P, et al. Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins. Mar Ecol. 2010;31: 21–50.
-
- Hogg MM, Tendal OS, Conway KW, Pomponi SA, van Soest RWM, Gutt J, et al. Deep-Sea sponge grounds: Reservoirs of biodiversity. Cambridge: UNEP-WMCM; 2010.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

