Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic α-synuclein mutant mouse model
- PMID: 26016857
- PMCID: PMC4449593
- DOI: 10.1186/s12974-015-0302-z
Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic α-synuclein mutant mouse model
Abstract
Background: The acupuncture or electroacupuncture (EA) shows the therapeutic effect on various neurodegenerative diseases. This effect was thought to be partially achieved by its ability to alleviate existing neuroinflammation and glial dysfunction. In this study, we systematically investigated the effect of EA on abnormal neurochemical changes and motor symptoms in a mouse neurodegenerative disease model.
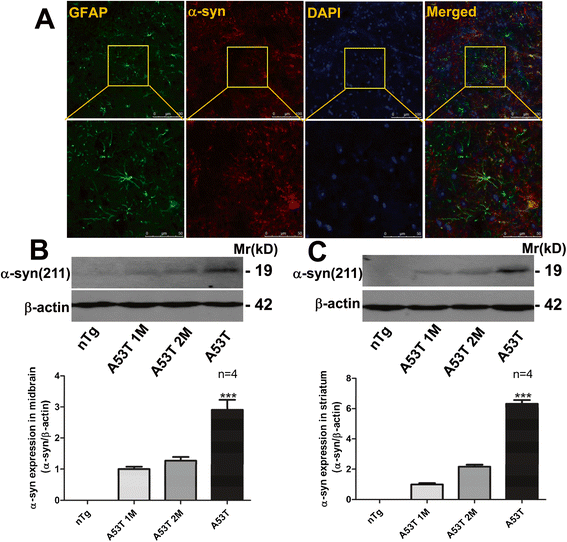
Methods: The transgenic mouse which expresses a mutant α-synuclein (α-syn) protein, A53T α-syn, in brain astrocytic cells was used. These mice exhibit extensive neuroinflammatory and motor phenotypes of neurodegenerative disorders. In this study, the effects of EA on these phenotypic changes were examined in these mice.
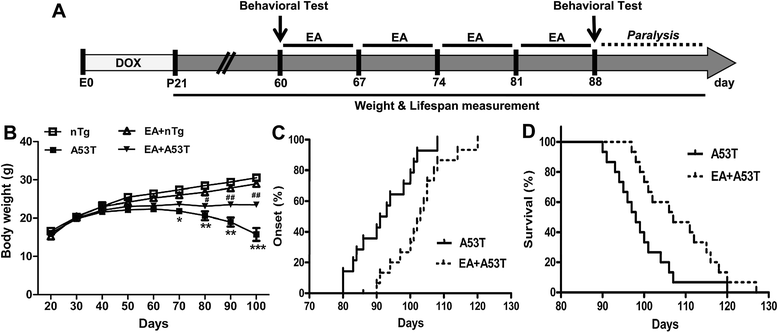
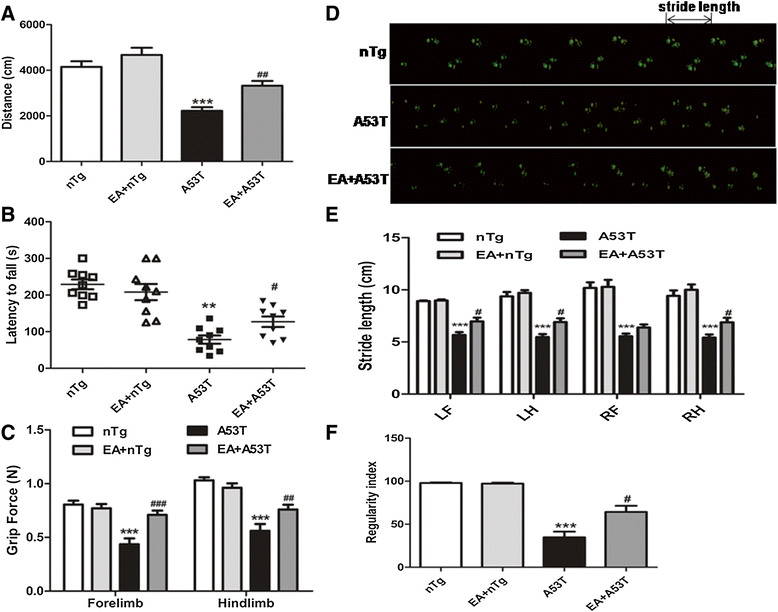
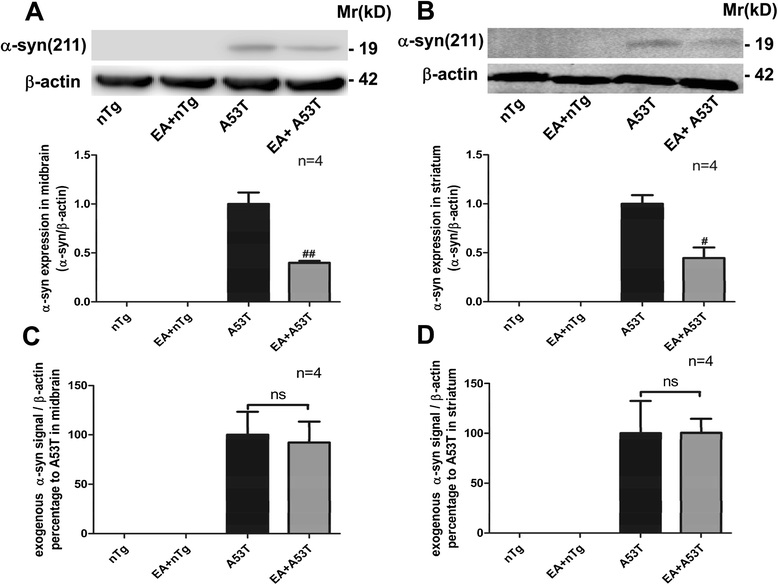
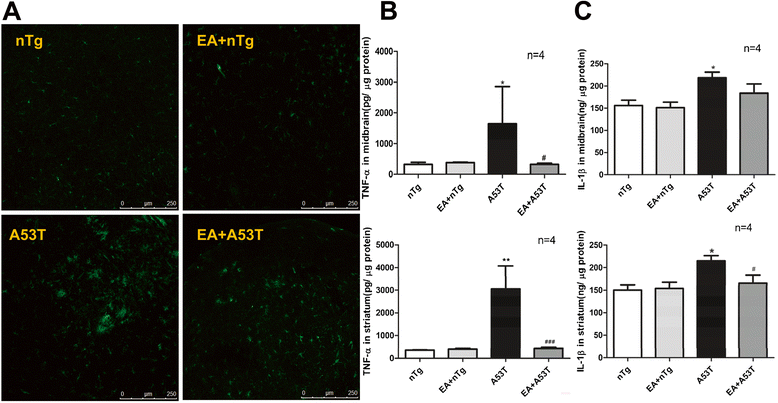
Results: EA improved the movement detected in multiple motor tests in A53T mutant mice. At the cellular level, EA significantly reduced the activation of microglia and prevented the loss of dopaminergic neurons in the midbrain and motor neurons in the spinal cord. At the molecular level, EA suppressed the abnormal elevation of proinflammatory factors (tumor necrosis factor-α and interleukin-1β) in the striatum and midbrain of A53T mice. In contrast, EA increased striatal and midbrain expression of a transcription factor, nuclear factor E2-related factor 2, and its downstream antioxidants (heme oxygenase-1 and glutamate-cysteine ligase modifier subunits).
Conclusions: These results suggest that EA possesses the ability to ameliorate mutant α-syn-induced motor abnormalities. This ability may be due to that EA enhances both anti-inflammatory and antioxidant activities and suppresses aberrant glial activation in the diseased sites of brains.
Figures



Similar articles
-
Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model.Glia. 2016 Dec;64(12):2219-2230. doi: 10.1002/glia.23058. Epub 2016 Sep 19. Glia. 2016. PMID: 27641725
-
Upregulation of neuronal zinc finger protein A20 expression is required for electroacupuncture to attenuate the cerebral inflammatory injury mediated by the nuclear factor-kB signaling pathway in cerebral ischemia/reperfusion rats.J Neuroinflammation. 2016 Oct 3;13(1):258. doi: 10.1186/s12974-016-0731-3. J Neuroinflammation. 2016. PMID: 27716383 Free PMC article.
-
Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies.Acta Neuropathol Commun. 2018 Jan 3;6(1):2. doi: 10.1186/s40478-017-0504-y. Acta Neuropathol Commun. 2018. PMID: 29298733 Free PMC article.
-
Electroacupuncture ameliorates neuroinflammation in animal models.Acupunct Med. 2022 Oct;40(5):474-483. doi: 10.1177/09645284221076515. Epub 2022 Mar 1. Acupunct Med. 2022. PMID: 35229660 Review.
-
Super-resolution imaging of alpha-synuclein polymorphisms and their potential role in neurodegeneration.Integr Biol (Camb). 2017 Mar 1;9(3):206-210. doi: 10.1039/c6ib00206d. Epub 2017 Feb 9. Integr Biol (Camb). 2017. PMID: 28180219 Review.
Cited by
-
Effects of Acupuncture on Neurological Disease in Clinical- and Animal-Based Research.Front Integr Neurosci. 2019 Aug 30;13:47. doi: 10.3389/fnint.2019.00047. eCollection 2019. Front Integr Neurosci. 2019. PMID: 31543763 Free PMC article. Review.
-
Electro-Acupuncture Ameliorated MPTP-Induced Parkinsonism in Mice via TrkB Neurotrophic Signaling.Front Neurosci. 2019 May 14;13:496. doi: 10.3389/fnins.2019.00496. eCollection 2019. Front Neurosci. 2019. PMID: 31156376 Free PMC article.
-
Acupuncture modulates the microbiota-gut-brain axis: a new strategy for Parkinson's disease treatment.Front Aging Neurosci. 2025 Aug 7;17:1640389. doi: 10.3389/fnagi.2025.1640389. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40851666 Free PMC article. Review.
-
Disease Stage-Associated Alterations in Learning and Memory through the Electroacupuncture Modulation of the Cortical Microglial M1/M2 Polarization in Mice with Alzheimer's Disease.Neural Plast. 2020 Aug 28;2020:8836173. doi: 10.1155/2020/8836173. eCollection 2020. Neural Plast. 2020. PMID: 32908486 Free PMC article.
-
Acupuncture for acute stroke.Cochrane Database Syst Rev. 2018 Mar 30;3(3):CD003317. doi: 10.1002/14651858.CD003317.pub3. Cochrane Database Syst Rev. 2018. PMID: 29607495 Free PMC article. Review.
References
-
- El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–7. - PubMed
-
- Jang A, Lee HJ, Suk JE, Jung JW, Kim KP, Lee SJ. Non-classical exocytosis of alpha-synuclein is sensitive to folding states and promoted under stress conditions. J Neurochem. 2010;113:1263–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

