Best (but oft-forgotten) practices: designing, analyzing, and reporting cluster randomized controlled trials
- PMID: 26016864
- PMCID: PMC4515862
- DOI: 10.3945/ajcn.114.105072
Best (but oft-forgotten) practices: designing, analyzing, and reporting cluster randomized controlled trials
Abstract
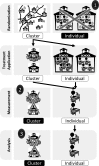
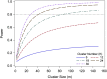
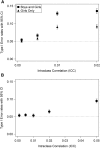
Cluster randomized controlled trials (cRCTs; also known as group randomized trials and community-randomized trials) are multilevel experiments in which units that are randomly assigned to experimental conditions are sets of grouped individuals, whereas outcomes are recorded at the individual level. In human cRCTs, clusters that are randomly assigned are typically families, classrooms, schools, worksites, or counties. With growing interest in community-based, public health, and policy interventions to reduce obesity or improve nutrition, the use of cRCTs has increased. Errors in the design, analysis, and interpretation of cRCTs are unfortunately all too common. This situation seems to stem in part from investigator confusion about how the unit of randomization affects causal inferences and the statistical procedures required for the valid estimation and testing of effects. In this article, we provide a brief introduction and overview of the importance of cRCTs and highlight and explain important considerations for the design, analysis, and reporting of cRCTs by using published examples.
Keywords: community randomized trial; group randomized trial; intraclass correlation coefficient; power analysis; reporting fidelity.
© 2015 American Society for Nutrition.
Figures
References
-
- Teerenstra S, Moerbeek M, van Achterberg T, Pelzer BJ, Borm GF. Sample size calculations for 3-level cluster randomized trials. Clin Trials 2008;5:486–95. - PubMed
-
- Murray DM. Design and analysis of group-randomized trials. Oxford (United Kingdom): Oxford University Press; 1998.
-
- Sim LJ, Parker L, Kumanyika SK. Bridging the evidence gap in obesity prevention: a framework to inform decision making. National Academies Press; 2010. - PubMed
-
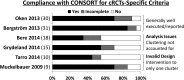
- Campbell MK, Piaggio G, Elbourne DR, Altman DG, Group C. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. - PubMed
-
- Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006;35:1292–300. - PubMed
APPENDIX A REFERENCES
-
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull 1979;86(2):420–8. - PubMed
-
- Turner RM, Prevost AT, Thompson SG. Allowing for imprecision of the intracluster correlation coefficient in the design of cluster randomized trials. Stat Med 2004;23(8):1195–214. - PubMed
-
- Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol 2006;35(5):1292–300. - PubMed
-
- Campbell MJ, Donner A, Klar N. Developments in cluster randomized trials and statistics in medicine. Stat Med 2007;26(1):2–19. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

