Cost-effectiveness of malaria diagnosis using rapid diagnostic tests compared to microscopy or clinical symptoms alone in Afghanistan
- PMID: 26016871
- PMCID: PMC4450447
- DOI: 10.1186/s12936-015-0696-1
Cost-effectiveness of malaria diagnosis using rapid diagnostic tests compared to microscopy or clinical symptoms alone in Afghanistan
Abstract
Background: Improving access to parasitological diagnosis of malaria is a central strategy for control and elimination of the disease. Malaria rapid diagnostic tests (RDTs) are relatively easy to perform and could be used in primary level clinics to increase coverage of diagnostics and improve treatment of malaria.
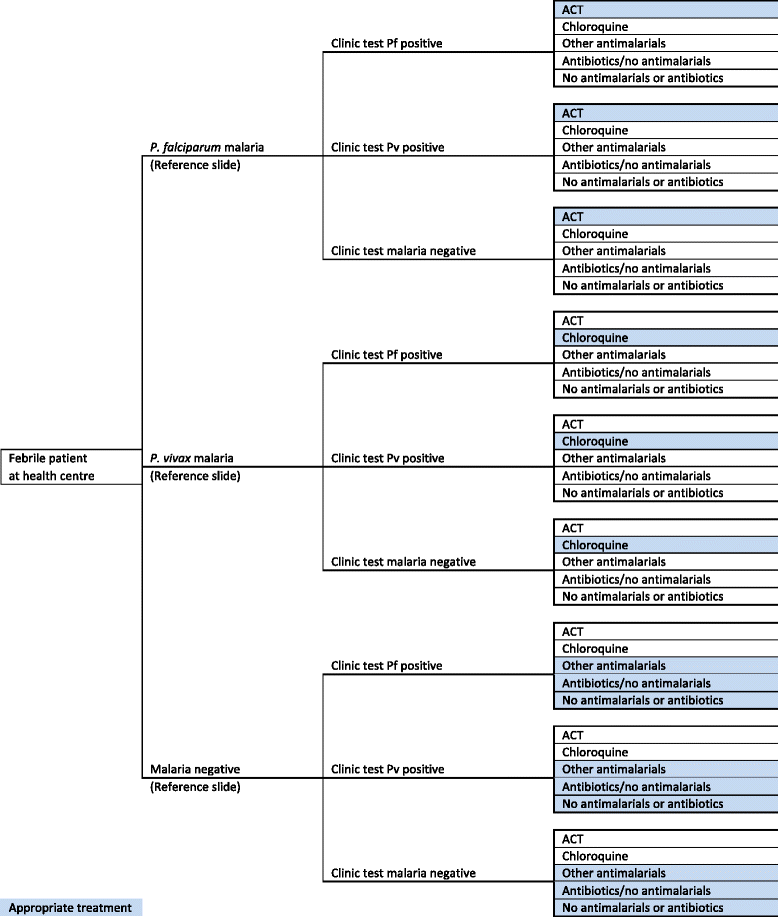
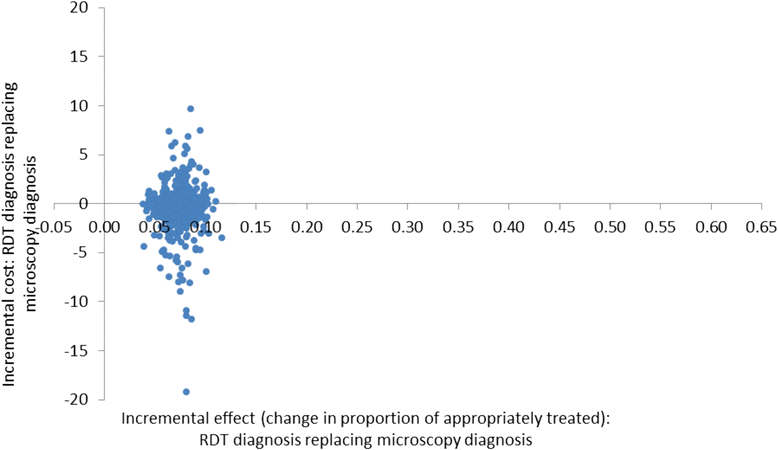
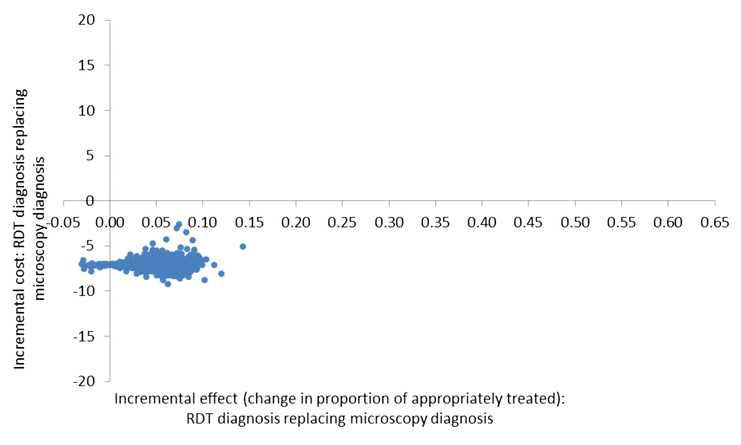
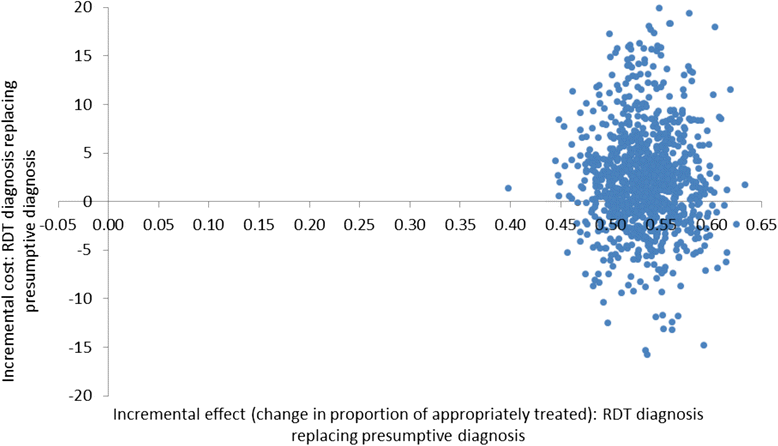
Methods: A cost-effectiveness analysis was undertaken of RDT-based diagnosis in public health sector facilities in Afghanistan comparing the societal and health sector costs of RDTs versus microscopy and RDTs versus clinical diagnosis in low and moderate transmission areas. The effect measure was 'appropriate treatment for malaria' defined using a reference diagnosis. Effects were obtained from a recent trial of RDTs in 22 public health centres with cost data collected directly from health centres and from patients enrolled in the trial. Decision models were used to compare the cost of RDT diagnosis versus the current diagnostic method in use at the clinic per appropriately treated case (incremental cost-effectiveness ratio, ICER).
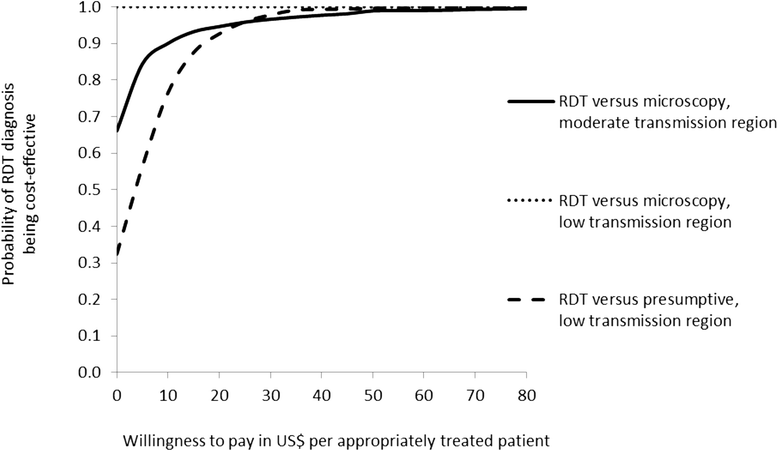
Results: RDT diagnosis of Plasmodium vivax and Plasmodium falciparum malaria in patients with uncomplicated febrile illness had higher effectiveness and lower cost compared to microscopy and was cost-effective across the moderate and low transmission settings. RDTs remained cost-effective when microscopy was used for other clinical purposes. In the low transmission setting, RDTs were much more effective than clinical diagnosis (65.2% (212/325) vs 12.5% (40/321)) but at an additional cost (ICER) of US$4.5 per appropriately treated patient including a health sector cost (ICER) of US$2.5 and household cost of US$2.0. Sensitivity analysis, which varied drug costs, indicated that RDTs would remain cost-effective if artemisinin combination therapy was used for treating both P. vivax and P. falciparum. Cost-effectiveness of microscopy relative to RDT is further reduced if the former is used exclusively for malaria diagnosis. In the health service setting of Afghanistan, RDTs are a cost-effective intervention compared to microscopy.
Conclusions: RDTs remain cost-effective across a range of drug costs and if microscopy is used for a range of diagnostic services. RDTs have significant advantages over clinical diagnosis with minor increases in the cost of service provision.
Trial registration: The trial was registered at ClinicalTrials.gov under identifier NCT00935688.
Figures
Similar articles
-
Performance of three multi-species rapid diagnostic tests for diagnosis of Plasmodium falciparum and Plasmodium vivax malaria in Oromia Regional State, Ethiopia.Malar J. 2010 Oct 27;9:297. doi: 10.1186/1475-2875-9-297. Malar J. 2010. PMID: 20979601 Free PMC article.
-
Use of HRP-2-based rapid diagnostic test for Plasmodium falciparum malaria: assessing accuracy and cost-effectiveness in the villages of Dielmo and Ndiop, Senegal.Malar J. 2010 Jun 4;9:153. doi: 10.1186/1475-2875-9-153. Malar J. 2010. PMID: 20525322 Free PMC article.
-
Should countries implementing an artemisinin-based combination malaria treatment policy also introduce rapid diagnostic tests?Malar J. 2008 Sep 15;7:176. doi: 10.1186/1475-2875-7-176. Malar J. 2008. PMID: 18793410 Free PMC article.
-
A review of the WHO malaria rapid diagnostic test product testing programme (2008-2018): performance, procurement and policy.Malar J. 2019 Dec 2;18(1):387. doi: 10.1186/s12936-019-3028-z. Malar J. 2019. PMID: 31791354 Free PMC article. Review.
-
Artemisinin combination therapy for vivax malaria.Lancet Infect Dis. 2010 Jun;10(6):405-16. doi: 10.1016/S1473-3099(10)70079-7. Lancet Infect Dis. 2010. PMID: 20510281 Free PMC article. Review.
Cited by
-
Modelling the cost-effectiveness of a rapid diagnostic test (IgMFA) for uncomplicated typhoid fever in Cambodia.PLoS Negl Trop Dis. 2018 Nov 19;12(11):e0006961. doi: 10.1371/journal.pntd.0006961. eCollection 2018 Nov. PLoS Negl Trop Dis. 2018. PMID: 30452445 Free PMC article.
-
Use of malaria rapid diagnostic tests by community health workers in Afghanistan: cluster randomised trial.BMC Med. 2017 Jul 7;15(1):124. doi: 10.1186/s12916-017-0891-8. BMC Med. 2017. PMID: 28683750 Free PMC article. Clinical Trial.
-
Epidemiology and Control of Plasmodium vivax in Afghanistan.Am J Trop Med Hyg. 2016 Dec 28;95(6 Suppl):72-77. doi: 10.4269/ajtmh.16-0172. Epub 2016 Oct 5. Am J Trop Med Hyg. 2016. PMID: 27708189 Free PMC article.
-
Transmission-blocking strategies: the roadmap from laboratory bench to the community.Malar J. 2016 Feb 18;15:95. doi: 10.1186/s12936-016-1163-3. Malar J. 2016. PMID: 26888537 Free PMC article. Review.
-
Costs and Cost-Effectiveness of Malaria Control Interventions: A Systematic Literature Review.Value Health. 2021 Aug;24(8):1213-1222. doi: 10.1016/j.jval.2021.01.013. Epub 2021 May 18. Value Health. 2021. PMID: 34372987 Free PMC article.
References
-
- WHO . Guidelines for the treatment of malaria. 2. Geneva: World Health Organization; 2010. - PubMed
-
- WHO . T3: Test. Treat. Track. Scaling up diagnostic testing, treatment and surveillance for malaria. Geneva: World Health Organization; 2012.
-
- WHO . World Malaria Report 2013. Geneva: World Health Organization; 2013.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

