Investigation of two novel approaches for detection of sulfate ion and methane dissolved in sediment pore water using Raman spectroscopy
- PMID: 26016919
- PMCID: PMC4507585
- DOI: 10.3390/s150612377
Investigation of two novel approaches for detection of sulfate ion and methane dissolved in sediment pore water using Raman spectroscopy
Abstract
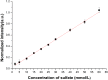
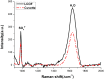
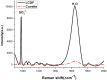
The levels of dissolved sulfate and methane are crucial indicators in the geochemical analysis of pore water. Compositional analysis of pore water samples obtained from sea trials was conducted using Raman spectroscopy. It was found that the concentration of SO42- in pore water samples decreases as the depth increases, while the expected Raman signal of methane has not been observed. A possible reason for this is that the methane escaped after sampling and the remaining concentration of methane is too low to be detected. To find more effective ways to analyze the composition of pore water, two novel approaches are proposed. One is based on Liquid Core Optical Fiber (LCOF) for detection of SO42-. The other one is an enrichment process for the detection of CH4. With the aid of LCOF, the Raman signal of SO42- is found to be enhanced over 10 times compared to that obtained by a conventional Raman setup. The enrichment process is also found to be effective in the investigation to the prepared sample of methane dissolved in water. By CCl4 extraction, methane at a concentration below 1.14 mmol/L has been detected by conventional Raman spectroscopy. All the obtained results suggest that the approach proposed in this paper has great potential to be developed as a sensor for SO42- and CH4 detection in pore water.
Keywords: CCl4 extraction; LCOF; Raman spectroscopy; methane; sulfate ion.
Figures






Similar articles
-
[The measurement of methane dissolved in water using raman spectroscopy assisted with CCl4 extraction].Guang Pu Xue Yu Guang Pu Fen Xi. 2012 Sep;32(9):2442-6. Guang Pu Xue Yu Guang Pu Fen Xi. 2012. PMID: 23240414 Chinese.
-
Feasibility Study on Quantitative Analysis of Sulfide Concentration and pH of Marine Sediment Pore Water via Raman Spectroscopy.Guang Pu Xue Yu Guang Pu Fen Xi. 2015 Mar;35(3):649-56. Guang Pu Xue Yu Guang Pu Fen Xi. 2015. PMID: 26117873
-
[Progress in Raman spectroscopic measurement of methane hydrate].Guang Pu Xue Yu Guang Pu Fen Xi. 2009 Sep;29(9):2457-61. Guang Pu Xue Yu Guang Pu Fen Xi. 2009. PMID: 19950652 Chinese.
-
Quantitative concentration measurements of creatinine dissolved in water and urine using Raman spectroscopy and a liquid core optical fiber.J Biomed Opt. 2005 May-Jun;10(3):031115. doi: 10.1117/1.1917842. J Biomed Opt. 2005. PMID: 16229640
-
Opportunities and challenges in the use of the Laser Methane Detector to monitor enteric methane emissions from ruminants.Animal. 2013 Jun;7 Suppl 2:394-400. doi: 10.1017/S1751731113000724. Animal. 2013. PMID: 23739480 Review.
Cited by
-
A Direct Bicarbonate Detection Method Based on a Near-Concentric Cavity-Enhanced Raman Spectroscopy System.Sensors (Basel). 2017 Dec 1;17(12):2784. doi: 10.3390/s17122784. Sensors (Basel). 2017. PMID: 29194357 Free PMC article.
-
Detection of contaminants in water supply: A review on state-of-the-art monitoring technologies and their applications.Sens Actuators B Chem. 2018 Feb;255:2657-2689. doi: 10.1016/j.snb.2017.09.078. Epub 2017 Sep 18. Sens Actuators B Chem. 2018. PMID: 32288249 Free PMC article. Review.
-
Selective Monitoring of Oxyanion Mixtures by a Flow System with Raman Detection.Sensors (Basel). 2018 Jul 8;18(7):2196. doi: 10.3390/s18072196. Sensors (Basel). 2018. PMID: 29986534 Free PMC article.
-
Concentration gradients in evaporating binary droplets probed by spatially resolved Raman and NMR spectroscopy.Proc Natl Acad Sci U S A. 2022 Apr 12;119(15):e2111989119. doi: 10.1073/pnas.2111989119. Epub 2022 Apr 4. Proc Natl Acad Sci U S A. 2022. PMID: 35377781 Free PMC article.
-
An Optical Fiber Sensor Based on Fluorescence Lifetime for the Determination of Sulfate Ions.Sensors (Basel). 2021 Feb 1;21(3):954. doi: 10.3390/s21030954. Sensors (Basel). 2021. PMID: 33535428 Free PMC article.
References
-
- Simoneit B.R.T., Schoell M., Dias R.F., de Auuino Neto F.R. Unusual carbon isotope compositions of biomarker hydrocarbons in a Permian tasmanite. Geochim. Cosmochim. Acta. 1993;57:4205–4211. doi: 10.1016/0016-7037(93)90316-O. - DOI
-
- Vairavamurthy M.A., Orr W.L., Manowitz B. ACS Symposium Series. American Chemical Society; Washington, DC, USA: 1995. Geochemical transformations of sedimentary sulfur: An introduction; pp. 1–15.
-
- Henrichs S.M., Reeburgh W.S. Anaerobic mineralization of marine sediment organic matter: Rates and the role of anaerobic processes in the oceanic carbon economy. Geomicrobiol. J. 1987;5:191–237. doi: 10.1080/01490458709385971. - DOI
-
- Jorgensen B.B., Kasten S. Sulfur Cycling and Methane Oxidation. In: Horst D.S., Matthias Z., editors. Marine Geochemistry. Springer; Berlin/Heidelberg, Germany: 2006. pp. 271–309.
LinkOut - more resources
Full Text Sources
Other Literature Sources

