Degradation of polyomavirus JC T-antigen by stress involves the LIP isoform of C/EBP
- PMID: 26017382
- PMCID: PMC4615132
- DOI: 10.1080/15384101.2015.1042631
Degradation of polyomavirus JC T-antigen by stress involves the LIP isoform of C/EBP
Abstract
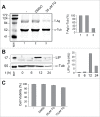
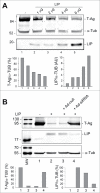
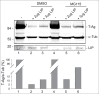
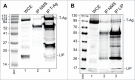
Endoplasmic reticulum (ER) stress is caused by the accumulation of misfolded or unfolded proteins in the lumen of the endoplasmic reticulum. CCAAT/enhancer binding proteins are one of the cellular proteins whose expression is upregulated during ER stress. Previously, we have identified C/EBPbeta isoforms, especially LIP, as a negative regulator of polyomavirus JC (JCV), the causative agent of the demyelinating disease progressive multifocal leukoencephalopathy (PML). Here, we show that the induction of ER stress by thapsigargin increase the expression of endogenous LIP and the degradation of JCV T-antigen in a JCV-transgenic mouse tumor cell line. Our results also revealed that overexpression of LIP significantly reduced the level of T-Ag and this effect is reversed upon siRNA-mediated silencing of LIP. Immunoprecipitation/Western blot experiments indicated that LIP interacts with T-antigen directly. Treatment of cells that overexpress LIP with MG115, a proteasome inhibitor, partially rescued LIP-mediated degradation of T-antigen. Our observations point to a role of LIP in ER stress regulation of T-antigen stability and may open a new avenue to study host-virus interaction during ER stress.
Keywords: CAAT/enhancer binding protein-beta; endoplasmic reticulum stress; large transforming antigen; liver-inhibitory isoform; polyomavirus JC.
Figures
Similar articles
-
IFNα and β Mediated JCPyV Suppression through C/EBPβ-LIP Isoform.Viruses. 2021 Sep 26;13(10):1937. doi: 10.3390/v13101937. Viruses. 2021. PMID: 34696366 Free PMC article.
-
Bag3-induced autophagy is associated with degradation of JCV oncoprotein, T-Ag.PLoS One. 2012;7(9):e45000. doi: 10.1371/journal.pone.0045000. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984599 Free PMC article.
-
Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer.J Exp Clin Cancer Res. 2018 Nov 27;37(1):286. doi: 10.1186/s13046-018-0967-0. J Exp Clin Cancer Res. 2018. PMID: 30482226 Free PMC article.
-
Expression of JC virus regulatory proteins in human cancer: potential mechanisms for tumourigenesis.Eur J Cancer. 2005 Nov;41(16):2537-48. doi: 10.1016/j.ejca.2005.08.019. Epub 2005 Oct 10. Eur J Cancer. 2005. PMID: 16219459 Review.
-
Overview of the cellular immunity against JC virus in progressive multifocal leukoencephalopathy.J Neurovirol. 2002 Dec;8 Suppl 2:59-65. doi: 10.1080/13550280290167894. J Neurovirol. 2002. PMID: 12491153 Review.
Cited by
-
The Oncogenic Roles of JC Virus T Antigen in Breast Carcinogenesis.Front Mol Biosci. 2021 Aug 12;8:687444. doi: 10.3389/fmolb.2021.687444. eCollection 2021. Front Mol Biosci. 2021. PMID: 34476239 Free PMC article.
-
The Brd4 acetyllysine-binding protein is involved in activation of polyomavirus JC.J Neurovirol. 2016 Oct;22(5):615-625. doi: 10.1007/s13365-016-0435-6. Epub 2016 Mar 23. J Neurovirol. 2016. PMID: 27007123 Free PMC article.
-
The oncogenic roles of JC polyomavirus in cancer.Front Oncol. 2022 Sep 23;12:976577. doi: 10.3389/fonc.2022.976577. eCollection 2022. Front Oncol. 2022. PMID: 36212474 Free PMC article. Review.
-
The DNA damage response promotes polyomavirus JC infection by nucleus to cytoplasm NF- kappaB activation.Virol J. 2017 Feb 15;14(1):31. doi: 10.1186/s12985-017-0707-7. Virol J. 2017. PMID: 28202068 Free PMC article.
-
The potential oncogenic effect of tissue-specific expression of JC polyoma T antigen in digestive epithelial cells.Transgenic Res. 2023 Aug;32(4):305-319. doi: 10.1007/s11248-023-00352-y. Epub 2023 May 29. Transgenic Res. 2023. PMID: 37247123 Free PMC article.
References
-
- Wollebo HS, White MK, Berger JR, Gordon J, Khalili K. Persistency and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol 2015; (In Press); 77(4):560-70; PMID:25623836; http://dx.doi.org/10.1002/ana.24371 - DOI - PMC - PubMed
-
- White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol 2009; 83:10846-56; PMID:19640999; http://dx.doi.org/10.1128/JVI.00542-09 - DOI - PMC - PubMed
-
- Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPbeta. Virus Res 2009; 146:97-106; PMID:19747512; http://dx.doi.org/10.1016/j.virusres.2009.09.005 - DOI - PMC - PubMed
-
- Wollebo HS, Melis S, Khalili K, Safak M, White MK. Cooperative roles of NF-κB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology 2012; 432:146-54; PMID:22749879; http://dx.doi.org/10.1016/j.virol.2012.06.010 - DOI - PMC - PubMed
-
- White MK, Khalili K. Polyomaviruses and human cancer: molecular mechanisms underlying patterns of tumorigenesis. Virology 2004; 324:1-16; PMID:15183048; http://dx.doi.org/10.1016/j.virol.2004.03.025 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
