Direct Detection by the Xpert MTB/RIF Assay and Characterization of Multi and Poly Drug-Resistant Tuberculosis in Guinea-Bissau, West Africa
- PMID: 26017968
- PMCID: PMC4446334
- DOI: 10.1371/journal.pone.0127536
Direct Detection by the Xpert MTB/RIF Assay and Characterization of Multi and Poly Drug-Resistant Tuberculosis in Guinea-Bissau, West Africa
Abstract
Background: This study aimed to evaluate the usefulness of the Xpert MTB/RIF assay for the rapid direct detection of M. tuberculosis complex (MTBC) strains and rifampicin resistance associated mutations in a resource-limited setting such as Guinea-Bissau and its implications in the management of tuberculosis (TB) and drug resistant tuberculosis, complementing the scarce information on resistance and genotypic diversity of MTBC strains in this West African country.
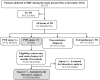
Methods and results: This cross-sectional prospective study included 100 consecutive TB patients with positive acid-fast smears at two months of anti-tuberculosis treatment or in a re-treatment situation, between May and December 2012. Resistance to rifampicin was detected using the GeneXpert system and the Xpert MTB/RIF assay. MTBC isolates obtained with the BACTEC MGIT 960 system were tested for susceptibility to first- and second-line anti-tuberculosis drugs. Overall, the prevalence of multidrug-resistant tuberculosis (MDR-TB) was found to be 9 cases. Of these, 67% (6 patients) of confirmed MDR-TB cases had no past history of TB treatment and 33% (3 patients) were previously treated cases. Extensively drug-resistant TB was not found. Molecular typing of the MDR-TB strains revealed recent transmission patterns of imported MDR strains.
Conclusions: The Xpert MTB/RIF assay was reliable for the detection of rifampicin resistant MTBC strains directly from sputum samples of patients undergoing first-line treatment for two months, being more trustworthy than the simple presence of acid-fast bacilli in the smear. Its implementation is technically simple, does not require specialized laboratory infrastructures and is suitable for resource-limited settings when a regular source of electricity and maintenance is available as well as financial and operation sustainability is guaranteed by the health authorities. A high prevalence of MDR-TB among patients at risk of MDR-TB after two months of first-line treatment was found, in support of the WHO recommendations for its use in the management of this risk group.
Conflict of interest statement
Figures

Similar articles
-
Diagnostic performance of the Xpert MTB/RIF assay for tuberculous lymphadenitis on fine needle aspirates from Ethiopia.Tuberculosis (Edinb). 2014 Sep;94(5):502-5. doi: 10.1016/j.tube.2014.05.002. Epub 2014 May 28. Tuberculosis (Edinb). 2014. PMID: 24931451
-
Utility of the REBA MTB-Rifa® assay for rapid detection of rifampicin resistant Mycobacterium tuberculosis.BMC Infect Dis. 2013 Oct 15;13:478. doi: 10.1186/1471-2334-13-478. BMC Infect Dis. 2013. PMID: 24128118 Free PMC article.
-
Efficacy of the Xpert MTB/RIF Assay in Multidrug-Resistant Tuberculosis.Microb Drug Resist. 2020 Aug;26(8):997-1004. doi: 10.1089/mdr.2019.0326. Epub 2020 Mar 16. Microb Drug Resist. 2020. PMID: 32181685
-
Xpert® MTB/RIF: Usefulness for the diagnosis of tuberculosis and resistance to rifampicin.Med Clin (Barc). 2017 Nov 9;149(9):399-405. doi: 10.1016/j.medcli.2017.06.007. Epub 2017 Jul 22. Med Clin (Barc). 2017. PMID: 28739268 Review. English, Spanish.
-
Rapid molecular diagnostics for multi-drug resistant tuberculosis in India.Expert Rev Anti Infect Ther. 2018 Mar;16(3):197-204. doi: 10.1080/14787210.2018.1438262. Epub 2018 Feb 12. Expert Rev Anti Infect Ther. 2018. PMID: 29406800 Review.
Cited by
-
Genetic diversity, transmission dynamics and drug resistance of Mycobacterium tuberculosis in Angola.Sci Rep. 2017 Feb 23;7:42814. doi: 10.1038/srep42814. Sci Rep. 2017. PMID: 28230095 Free PMC article.
-
Feasibility and Effectiveness of Tuberculosis Active Case-Finding among Children Living with Tuberculosis Relatives: a Cross-Sectional Study in Guinea-Bissau.Mediterr J Hematol Infect Dis. 2017 Oct 15;9(1):e2017059. doi: 10.4084/MJHID.2017.059. eCollection 2017. Mediterr J Hematol Infect Dis. 2017. PMID: 29181136 Free PMC article.
-
Experimental Evidence for Limited in vivo Virulence of Mycobacterium africanum.Front Microbiol. 2019 Sep 10;10:2102. doi: 10.3389/fmicb.2019.02102. eCollection 2019. Front Microbiol. 2019. PMID: 31552007 Free PMC article.
-
Distribution of HbS Allele and Haplotypes in a Multi-Ethnic Population of Guinea Bissau, West Africa: Implications for Public Health Screening.Front Pediatr. 2022 Apr 7;10:826262. doi: 10.3389/fped.2022.826262. eCollection 2022. Front Pediatr. 2022. PMID: 35463879 Free PMC article.
-
Emergence of multidrug-resistant Mycobacterium tuberculosis of the Beijing lineage in Portugal and Guinea-Bissau: a snapshot of moving clones by whole-genome sequencing.Emerg Microbes Infect. 2020 Dec;9(1):1342-1353. doi: 10.1080/22221751.2020.1774425. Emerg Microbes Infect. 2020. PMID: 32538300 Free PMC article.
References
-
- World Health Organization. Global tuberculosis report 2014. WHO/HTM/TB/2014.08. Geneva, Switzerland: World Health Organization; 2014.
-
- World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis WHO/HTM/TB/2011.6. Geneva, Switzerland: World Health Organization; 2011.
-
- Atun RA, Lebcir R, Drobniewski F, Coker RJ. Impact of an effective multidrug-resistant tuberculosis control programme in the setting of an immature HIV epidemic: system dynamics simulation model. Int J STD AIDS. 2005; 16(8): 560–570. - PubMed
-
- Drobniewski FA, Balabanova YM, Ruddy MC, Graham C, Kuznetzov SI, Gusarova GI, et al. Tuberculosis, HIV seroprevalence and intravenous and drug abuse in prisoners. Eur Respir J. 2008; 26(2): 298–304. - PubMed
-
- World Health Organization. Global tuberculosis report 2013. WHO/HTM/TB/2013.11. Geneva, Switzerland: World Health Organization; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

