Nicotine and the adolescent brain
- PMID: 26018031
- PMCID: PMC4560573
- DOI: 10.1113/JP270492
Nicotine and the adolescent brain
Abstract
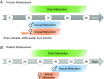
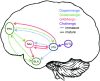
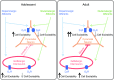
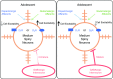
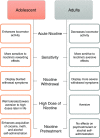
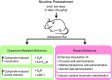
Adolescence encompasses a sensitive developmental period of enhanced clinical vulnerability to nicotine, tobacco, and e-cigarettes. While there are sociocultural influences, data at preclinical and clinical levels indicate that this adolescent sensitivity has strong neurobiological underpinnings. Although definitions of adolescence vary, the hallmark of this period is a profound reorganization of brain regions necessary for mature cognitive and executive function, working memory, reward processing, emotional regulation, and motivated behavior. Regulating critical facets of brain maturation are nicotinic acetylcholine receptors (nAChRs). However, perturbations of cholinergic systems during this time with nicotine, via tobacco or e-cigarettes, have unique consequences on adolescent development. In this review, we highlight recent clinical and preclinical data examining the adolescent brain's distinct neurobiology and unique sensitivity to nicotine. First, we discuss what defines adolescence before reviewing normative structural and neurochemical alterations that persist until early adulthood, with an emphasis on dopaminergic systems. We review how acute exposure to nicotine impacts brain development and how drug responses differ from those seen in adults. Finally, we discuss the persistent alterations in neuronal signaling and cognitive function that result from chronic nicotine exposure, while highlighting a low dose, semi-chronic exposure paradigm that may better model adolescent tobacco use. We argue that nicotine exposure, increasingly occurring as a result of e-cigarette use, may induce epigenetic changes that sensitize the brain to other drugs and prime it for future substance abuse.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures
References
-
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. - PubMed
-
- Aksglaede L, Sørensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics. 2009;123:e932–939. - PubMed
-
- Albuquerque EX, Pereira EF, Braga MF, Alkondon M. Contribution of nicotinic receptors to the function of synapses in the central nervous system: the action of choline as a selective agonist of α7 receptors. J Physiol Paris. 1998;92:309–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

