Prion-Protein-interacting Amyloid-β Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models
- PMID: 26018073
- PMCID: PMC4498078
- DOI: 10.1074/jbc.M115.643577
Prion-Protein-interacting Amyloid-β Oligomers of High Molecular Weight Are Tightly Correlated with Memory Impairment in Multiple Alzheimer Mouse Models
Abstract
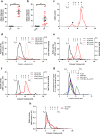
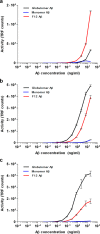
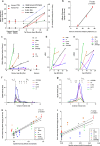
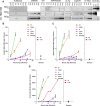
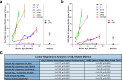
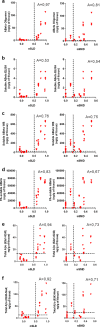
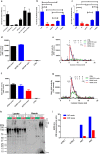
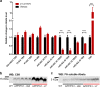
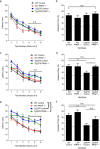
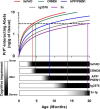
Alzheimer disease (AD) is characterized by amyloid-β accumulation, with soluble oligomers (Aβo) being the most synaptotoxic. However, the multivalent and unstable nature of Aβo limits molecular characterization and hinders research reproducibility. Here, we characterized multiple Aβo forms throughout the life span of various AD mice and in post-mortem human brain. Aβo exists in several populations, where prion protein (PrP(C))-interacting Aβo is a high molecular weight Aβ assembly present in multiple mice and humans with AD. Levels of PrP(C)-interacting Aβo match closely with mouse memory and are equal or superior to other Aβ measures in predicting behavioral impairment. However, Aβo metrics vary considerably between mouse strains. Deleting PrP(C) expression in mice with relatively low PrP(C)-interacting Aβo (Tg2576) results in partial rescue of cognitive performance as opposed to complete recovery in animals with a high percentage of PrP(C)-interacting Aβo (APP/PSEN1). These findings highlight the relative contributions and interplay of Aβo forms in AD.
Keywords: Alzheimer disease; amyloid-beta (AB); learn; ligand-binding protein; memory; oligomer; prion; transgenic mice.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Mayeux R. (2003) Epidemiology of neurodegeneration. Annu. Rev. Neurosci. 26, 81–104 - PubMed
-
- Braak H., Braak E. (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 - PubMed
-
- Blennow K., de Leon M. J., Zetterberg H. (2006) Alzheimer's disease. Lancet 368, 387–403 - PubMed
-
- Arendt T. (2009) Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 118, 167–179 - PubMed
-
- Prince M., Bryce R., Albanese E., Wimo A., Ribeiro W., Ferri C. P. (2013) The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's Dementia 9, 63–75 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

