Adaptive Engineering of Phytochelatin-based Heavy Metal Tolerance
- PMID: 26018077
- PMCID: PMC4498070
- DOI: 10.1074/jbc.M115.652123
Adaptive Engineering of Phytochelatin-based Heavy Metal Tolerance
Abstract
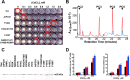
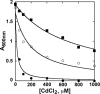
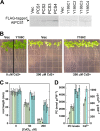
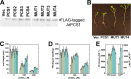
Metabolic engineering approaches are increasingly employed for environmental applications. Because phytochelatins (PC) protect plants from heavy metal toxicity, strategies directed at manipulating the biosynthesis of these peptides hold promise for the remediation of soils and groundwaters contaminated with heavy metals. Directed evolution of Arabidopsis thaliana phytochelatin synthase (AtPCS1) yields mutants that confer levels of cadmium tolerance and accumulation greater than expression of the wild-type enzyme in Saccharomyces cerevisiae, Arabidopsis, or Brassica juncea. Surprisingly, the AtPCS1 mutants that enhance cadmium tolerance and accumulation are catalytically less efficient than wild-type enzyme. Metabolite analyses indicate that transformation with AtPCS1, but not with the mutant variants, decreases the levels of the PC precursors, glutathione and γ-glutamylcysteine, upon exposure to cadmium. Selection of AtPCS1 variants with diminished catalytic activity alleviates depletion of these metabolites, which maintains redox homeostasis while supporting PC synthesis during cadmium exposure. These results emphasize the importance of metabolic context for pathway engineering and broaden the range of tools available for environmental remediation.
Keywords: enzyme catalysis; heavy metal tolerance; metabolic engineering; plant biochemistry; plant molecular biology; protein engineering.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Johnson M. D., Kenney N., Stoica A., Hilakivi-Clarke L., Singh B., Chepko G., Clarke R., Sholler P. F., Lirio A. A., Foss C., Reiter R., Trock B., Paik S., Martin M. B. (2003) Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nature Med. 9, 1081–1084 - PubMed
-
- Salt D. E., Smith R. D., Raskin I. (1998) Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 643–668 - PubMed
-
- Lassner M., Bedbrook J. (2001) Directed molecular evolution in plant improvement. Curr. Opin. Plant Biol. 4, 152–156 - PubMed
-
- Bizily S. P., Rugh C. L., Meagher R. B. (2000) Phytodetoxification of hazardous organomercurials by genetically engineered plants. Nat. Biotechnol. 18, 213–217 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

