Promotion of Endoplasmic Reticulum-Associated Degradation of Procathepsin D by Human Herpesvirus 8-Encoded Viral Interleukin-6
- PMID: 26018151
- PMCID: PMC4505649
- DOI: 10.1128/JVI.00375-15
Promotion of Endoplasmic Reticulum-Associated Degradation of Procathepsin D by Human Herpesvirus 8-Encoded Viral Interleukin-6
Abstract
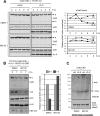
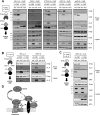
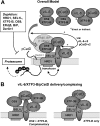
The interleukin-6 homologue (viral interleukin-6 [vIL-6]) of human herpesvirus 8 is implicated in viral pathogenesis due to its proproliferative, inflammatory, and angiogenic properties, effected through gp130 receptor signaling. In primary effusion lymphoma (PEL) cells, vIL-6 is expressed latently and is essential for normal cell growth and viability. This is mediated partly via suppression of proapoptotic cathepsin D (CatD) via cocomplexing of the endoplasmic reticulum (ER)-localized CatD precursor, pro-CatD (pCatD), and vIL-6 with the previously uncharacterized ER membrane protein vitamin K epoxide reductase complex subunit 1 variant 2 (VKORC1v2). vIL-6 suppression of CatD occurs also during reactivated productive replication in PEL cells and is likely to contribute to proreplication functions of vIL-6. Here, we report that vIL-6 suppresses CatD through vIL-6, VKORC1v2, and pCatD association with components of the ER-associated degradation (ERAD) machinery. In transfected cells, expression of vIL-6 along with CatD led to proteasome-dependent (inhibitor-sensitive) decreases in CatD levels and the promotion of pCatD polyubiquitination. Depletion of particular ERAD-associated isomerases, lectins, and translocon components, including ERAD E3 ubiquitin ligase HRD1, diminished suppression of CatD by vIL-6. Coprecipitation assays identified direct or indirect interactions of VKORC1v2, vIL-6, and pCatD with translocon proteins (SEL1L and/or HRD1) and ERAD-associated lectins OS9 and XTP3-B. Endogenous CatD expression in PEL cells was increased by depletion of ERAD components, and suppression of CatD by vIL-6 overexpression in PEL cells was dependent on HRD1. Our data reveal a new mechanism of ER-localized vIL-6 activity and further characterize VKORC1v2 function.
Importance: Human herpesvirus 8 (HHV-8) viral interleukin-6 (vIL-6), unlike cellular IL-6 proteins, is secreted inefficiently and sequestered mainly in the endoplasmic reticulum (ER), from where it can signal through the gp130 receptor. We have recently reported that vIL-6 also associates with a novel membrane protein termed vitamin K epoxide reductase complex subunit 1 variant 2 (VKORC1v2) and mediates suppression of VKORC1v2-cointeracting cathepsin D, a stress-released proapoptotic protein negatively impacting HHV-8 latently infected primary effusion lymphoma (PEL) cell viability and reactivated virus productive replication. Here, we have examined the mechanistic basis of the VKORC1v2-vIL-6 interaction-dependent suppression of cathepsin D and have found that this novel activity of vIL-6 is mediated through coassociation of VKORC1v2, procathepsin D, and vIL-6 with components of the ER-associated degradation (ERAD) machinery. Our findings provide information of significance for potential antiviral and therapeutic targeting of VKORC1v2-mediated vIL-6 activities and also indicate the nature of VKORC1v2 function in normal cell biology.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Arvanitakis L, Mesri EA, Nador RG, Said JW, Asch AS, Knowles DM, Cesarman E. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648–2654. - PubMed
-
- Chang Y, Moore PS. 1996. Kaposi's sarcoma (KS)-associated herpesvirus and its role in KS. Infect Agents Dis 5:215–222. - PubMed
-
- Gaidano G, Pastore C, Gloghini A, Volpe G, Capello D, Polito P, Vaccher E, Tirelli U, Saglio G, Carbone A. 1997. Human herpesvirus type-8 (HHV-8) in haematopoietic neoplasia. Leuk Lymphoma 24:257–266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

