Selective recruitment of nuclear factors to productively replicating herpes simplex virus genomes
- PMID: 26018390
- PMCID: PMC4446364
- DOI: 10.1371/journal.ppat.1004939
Selective recruitment of nuclear factors to productively replicating herpes simplex virus genomes
Abstract
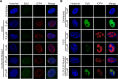
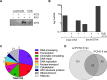
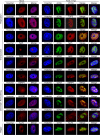
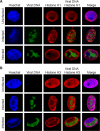
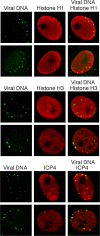
Much of the HSV-1 life cycle is carried out in the cell nucleus, including the expression, replication, repair, and packaging of viral genomes. Viral proteins, as well as cellular factors, play essential roles in these processes. Isolation of proteins on nascent DNA (iPOND) was developed to label and purify cellular replication forks. We adapted aspects of this method to label viral genomes to both image, and purify replicating HSV-1 genomes for the identification of associated proteins. Many viral and cellular factors were enriched on viral genomes, including factors that mediate DNA replication, repair, chromatin remodeling, transcription, and RNA processing. As infection proceeded, packaging and structural components were enriched to a greater extent. Among the more abundant proteins that copurified with genomes were the viral transcription factor ICP4 and the replication protein ICP8. Furthermore, all seven viral replication proteins were enriched on viral genomes, along with cellular PCNA and topoisomerases, while other cellular replication proteins were not detected. The chromatin-remodeling complexes present on viral genomes included the INO80, SWI/SNF, NURD, and FACT complexes, which may prevent chromatinization of the genome. Consistent with this conclusion, histones were not readily recovered with purified viral genomes, and imaging studies revealed an underrepresentation of histones on viral genomes. RNA polymerase II, the mediator complex, TFIID, TFIIH, and several other transcriptional activators and repressors were also affinity purified with viral DNA. The presence of INO80, NURD, SWI/SNF, mediator, TFIID, and TFIIH components is consistent with previous studies in which these complexes copurified with ICP4. Therefore, ICP4 is likely involved in the recruitment of these key cellular chromatin remodeling and transcription factors to viral genomes. Taken together, iPOND is a valuable method for the study of viral genome dynamics during infection and provides a comprehensive view of how HSV-1 selectively utilizes cellular resources.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, et al. (1988) The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol 69: 1531–1574. - PubMed
-
- Shahin V, Hafezi W, Oberleithner H, Ludwig Y, Windoffer B, et al. (2006) The genome of HSV-1 translocates through the nuclear pore as a condensed rod-like structure. J Cell Sci 119: 23–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

