Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection
- PMID: 26018856
- PMCID: PMC4790688
Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection
Abstract
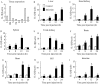
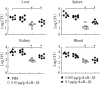
Interleukin 1β (IL-1β), the first interleukin to be characterized, plays a key role in regulating the immune response. In this study, we determined the cDNA and genomic DNA sequences of the IL-1β gene from the large yellow croaker, Larimichthys crocea. Phylogenetic analysis indicated that the IL-1β (LcIL-1β) gene was most closely related to that of European seabass (Dicentrarchus labrax), sharing 67.8% amino acid identity. In healthy large yellow croaker, LcIL-1β transcription was detected in all tested tissues, with the highest level found in the head kidney. Upon Vibrio alginolyticus infection, LcIL-1β transcription in all tested tissues was significantly upregulated. Intraperitoneal injection of recombinant LcIL-1β (rLcIL-1β) improved the survival rate and reduced the tissue bacterial load after V. alginolyticus infection. In addition, rLcIL-1β induced monocytes/macrophages (MO/MΦ) chemotaxis and increased phagocytosis and bactericidal activity in vitro. These results suggest that LcIL-1β plays an important role in the large yellow croaker immune response against V. alginolyticus.
Keywords: Interleukin 1β; Large yellow croaker; Monocytes/ macrophages; Survival rate; Vibrio alginolyticus.
Figures





References
-
- Angosto D, Montero J, López-Muñoz A, Alcaraz-Pérez F, Bird S, Sarropoulou E, Abellán E, Meseguer J, Sepulcre MP, Mulero V.2013. Identification and functional characterization of a new IL-1 family member, IL-1Fm2, in most evolutionarily advanced fish.Innate Immunity, 20(5): 133-500. - PubMed
- Angosto D, Montero J, López-Muñoz A, Alcaraz-Pérez F, Bird S, Sarropoulou E, Abellán E, Meseguer J, Sepulcre MP, Mulero V.2013. Identification and functional characterization of a new IL-1 family member, IL-1Fm2, in most evolutionarily advanced fish.Innate Immunity, 20(5): 141-500. - PubMed
-
- Bird PI, Trapani JA, Villadangos JA.2009. Endolysosomal proteases and their inhibitors in immunity.Nature Reviews Immunology, 9(12): 871-882. - PubMed
-
- Buonocore F, Forlenza M, Randelli E, Benedetti S, Bossù P, Meloni S, Secombes CJ, Mazzini M, Scapigliati G.2005. Biological activity of sea bass (Dicentrarchus labrax L.) recombinant interleukin-1β.Marine Biotechnology, 7(6): 609-617. - PubMed
-
- Cai ZH, Song LS, Gao CP, Wu LT, Qiu LH.2004. Molecular cloning and expression of interleukin 1 beta (IL-1β) from red sea bream (Pagrus major).Progress in Natural Science, 14(5): 396-402.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
