Discovery and Characterization of Nonpeptidyl Agonists of the Tissue-Protective Erythropoietin Receptor
- PMID: 26018904
- PMCID: PMC4518087
- DOI: 10.1124/mol.115.098400
Discovery and Characterization of Nonpeptidyl Agonists of the Tissue-Protective Erythropoietin Receptor
Abstract
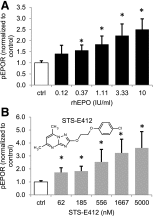
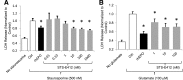
Erythropoietin (EPO) and its receptor are expressed in a wide variety of tissues, including the central nervous system. Local expression of both EPO and its receptor is upregulated upon injury or stress and plays a role in tissue homeostasis and cytoprotection. High-dose systemic administration or local injection of recombinant human EPO has demonstrated encouraging results in several models of tissue protection and organ injury, while poor tissue availability of the protein limits its efficacy. Here, we describe the discovery and characterization of the nonpeptidyl compound STS-E412 (2-[2-(4-chlorophenoxy)ethoxy]-5,7-dimethyl-[1,2,4]triazolo[1,5-a]pyrimidine), which selectively activates the tissue-protective EPO receptor, comprising an EPO receptor subunit (EPOR) and the common β-chain (CD131). STS-E412 triggered EPO receptor phosphorylation in human neuronal cells. STS-E412 also increased phosphorylation of EPOR, CD131, and the EPO-associated signaling molecules JAK2 and AKT in HEK293 transfectants expressing EPOR and CD131. At low nanomolar concentrations, STS-E412 provided EPO-like cytoprotective effects in primary neuronal cells and renal proximal tubular epithelial cells. The receptor selectivity of STS-E412 was confirmed by a lack of phosphorylation of the EPOR/EPOR homodimer, lack of activity in off-target selectivity screening, and lack of functional effects in erythroleukemia cell line TF-1 and CD34(+) progenitor cells. Permeability through artificial membranes and Caco-2 cell monolayers in vitro and penetrance across the blood-brain barrier in vivo suggest potential for central nervous system availability of the compound. To our knowledge, STS-E412 is the first nonpeptidyl, selective activator of the tissue-protective EPOR/CD131 receptor. Further evaluation of the potential of STS-E412 in central nervous system diseases and organ protection is warranted.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures


References
-
- Abe M, Suzuki K, Sakata C, Sugasawa K, Hirayama F, Koga Y, Kawasaki T, Naganuma S, Itoh H. (2011) Pharmacological profile of AS1670542, a novel orally-active human thrombopoietin receptor agonist. Eur J Pharmacol 650:58–63. - PubMed
-
- Assaraf MI, Diaz Z, Liberman A, Miller WHJ, Jr, Arvanitakis Z, Li Y, Bennett DA, Schipper HM. (2007) Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol 66:389–398. - PubMed
-
- Bennis Y, Sarlon-Bartoli G, Guillet B, Lucas L, Pellegrini L, Velly L, Blot-Chabaud M, Dignat-Georges F, Sabatier F, Pisano P. (2012) Priming of late endothelial progenitor cells with erythropoietin before transplantation requires the CD131 receptor subunit and enhances their angiogenic potential. J Thromb Haemost 10:1914–1928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

