Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities
- PMID: 26019168
- PMCID: PMC4648464
- DOI: 10.1093/aob/mcv054
Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities
Abstract
Background: Plants are hotbeds for parasites such as arthropod herbivores, which acquire nutrients and energy from their hosts in order to grow and reproduce. Hence plants are selected to evolve resistance, which in turn selects for herbivores that can cope with this resistance. To preserve their fitness when attacked by herbivores, plants can employ complex strategies that include reallocation of resources and the production of defensive metabolites and structures. Plant defences can be either prefabricated or be produced only upon attack. Those that are ready-made are referred to as constitutive defences. Some constitutive defences are operational at any time while others require activation. Defences produced only when herbivores are present are referred to as induced defences. These can be established via de novo biosynthesis of defensive substances or via modifications of prefabricated substances and consequently these are active only when needed. Inducibility of defence may serve to save energy and to prevent self-intoxication but also implies that there is a delay in these defences becoming operational. Induced defences can be characterized by alterations in plant morphology and molecular chemistry and are associated with a decrease in herbivore performance. These alterations are set in motion by signals generated by herbivores. Finally, a subset of induced metabolites are released into the air as volatiles and function as a beacon for foraging natural enemies searching for prey, and this is referred to as induced indirect defence.
Scope: The objective of this review is to evaluate (1) which strategies plants have evolved to cope with herbivores and (2) which traits herbivores have evolved that enable them to counter these defences. The primary focus is on the induction and suppression of plant defences and the review outlines how the palette of traits that determine induction/suppression of, and resistance/susceptibility of herbivores to, plant defences can give rise to exploitative competition and facilitation within ecological communities "inhabiting" a plant.
Conclusions: Herbivores have evolved diverse strategies, which are not mutually exclusive, to decrease the negative effects of plant defences in order to maximize the conversion of plant material into offspring. Numerous adaptations have been found in herbivores, enabling them to dismantle or bypass defensive barriers, to avoid tissues with relatively high levels of defensive chemicals or to metabolize these chemicals once ingested. In addition, some herbivores interfere with the onset or completion of induced plant defences, resulting in the plant's resistance being partly or fully suppressed. The ability to suppress induced plant defences appears to occur across plant parasites from different kingdoms, including herbivorous arthropods, and there is remarkable diversity in suppression mechanisms. Suppression may strongly affect the structure of the food web, because the ability to suppress the activation of defences of a communal host may facilitate competitors, whereas the ability of a herbivore to cope with activated plant defences will not. Further characterization of the mechanisms and traits that give rise to suppression of plant defences will enable us to determine their role in shaping direct and indirect interactions in food webs and the extent to which these determine the coexistence and persistence of species.
Keywords: Herbivory; adaptation; community interactions; detoxification; facilitation; herbivore; induction; jasmonate; manipulation; plant defence; plant–animal interaction; resistance; salicylate; sequestration; suppression.
© The Author 2015. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


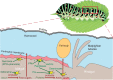

References
-
- Abe H, Tomitaka Y, Shimoda T, et al. 2012. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant & Cell Physiology 53: 204–212. - PubMed
-
- Adie B, Chico JM, Rubio-Somoza I, Solano R. 2007. Modulation of plant defenses by ethylene. Journal of Plant Growth Regulation 26: 160–177.
-
- Afshar K, Fikru-Dube F, Najafabadi HS, et al. 2013. Insights into the insect salivary gland proteome: diet-associated changes in caterpillar labial salivary proteins. Journal of Insect Physiology 59: 351–366. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

