SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma
- PMID: 26019222
- PMCID: PMC4503260
- DOI: 10.1126/scitranslmed.aaa8103
SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma
Abstract
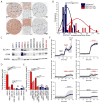
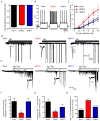
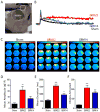
Glioma is the most common malignant primary brain tumor. Its rapid growth is aided by tumor-mediated glutamate release, creating peritumoral excitotoxic cell death and vacating space for tumor expansion. Glioma glutamate release may also be responsible for seizures, which complicate the clinical course for many patients and are often the presenting symptom. A hypothesized glutamate release pathway is the cystine/glutamate transporter System xc (-) (SXC), responsible for the cellular synthesis of glutathione (GSH). However, the relationship of SXC-mediated glutamate release, seizures, and tumor growth remains unclear. Probing expression of SLC7A11/xCT, the catalytic subunit of SXC, in patient and mouse-propagated tissues, we found that ~50% of patient tumors have elevated SLC7A11 expression. Compared with tumors lacking this transporter, in vivo propagated and intracranially implanted SLC7A11-expressing tumors grew faster, produced pronounced peritumoral glutamate excitotoxicity, induced seizures, and shortened overall survival. In agreement with animal data, increased SLC7A11 expression predicted shorter patient survival according to genomic data in the REMBRANDT (National Institutes of Health Repository for Molecular Brain Neoplasia Data) database. In a clinical pilot study, we used magnetic resonance spectroscopy to determine SXC-mediated glutamate release by measuring acute changes in glutamate after administration of the U.S. Food and Drug Administration-approved SXC inhibitor, sulfasalazine (SAS). In nine glioma patients with biopsy-confirmed SXC expression, we found that expression positively correlates with glutamate release, which is acutely inhibited with oral SAS. These data suggest that SXC is the major pathway for glutamate release from gliomas and that SLC7A11 expression predicts accelerated growth and tumor-associated seizures.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures



Comment in
-
Excitotoxic SLC7A11 Expression Is a Marker of Poor Glioblastoma Survival and a Potential Therapeutic Target.Neurosurgery. 2015 Dec;77(6):N16-7. doi: 10.1227/01.neu.0000473809.76102.ee. Neurosurgery. 2015. PMID: 26584321 No abstract available.
-
The role of glutamate transport and SLC7A11 expression in tumor-associate seizures and survival in patients with malignant gliomas.Ann Transl Med. 2016 Oct;4(Suppl 1):S18. doi: 10.21037/atm.2016.10.23. Ann Transl Med. 2016. PMID: 27867986 Free PMC article. No abstract available.
-
Glioma, glutamate (SLC7A11) and seizures-a commentary.Ann Transl Med. 2017 May;5(10):214. doi: 10.21037/atm.2017.02.18. Ann Transl Med. 2017. PMID: 28603729 Free PMC article. No abstract available.
References
-
- Marcus HJ, Carpenter KL, Price SJ, Hutchinson PJ. In vivo assessment of high-grade glioma biochemistry using microdialysis: a study of energy-related molecules, growth factors and cytokines. J Neurooncol. 2010;97:11–23. - PubMed
-
- Ishiuchi S, et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8:971–978. - PubMed
-
- Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress in neurobiology. 2014;115:157–188. - PubMed
-
- Bonova P, Burda J, Danielisova V, Nemethova M, Gottlieb M. Delayed postconditioning reduces post-ischemic glutamate level and improves protein synthesis in brain. Neurochemistry international. 2013;62:854–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1F31NS074597/NS/NINDS NIH HHS/United States
- TL1 RR025775/RR/NCRR NIH HHS/United States
- 5R01NS031234/NS/NINDS NIH HHS/United States
- T32 GM008362/GM/NIGMS NIH HHS/United States
- P30-HD38985/HD/NICHD NIH HHS/United States
- 2T32NS048039/NS/NINDS NIH HHS/United States
- R01 NS052634/NS/NINDS NIH HHS/United States
- T32GM008361/GM/NIGMS NIH HHS/United States
- R01 NS036692/NS/NINDS NIH HHS/United States
- T32 GM008361/GM/NIGMS NIH HHS/United States
- 5TL1RR025775-04/RR/NCRR NIH HHS/United States
- P30 NS047466/NS/NINDS NIH HHS/United States
- R01 NS082851/NS/NINDS NIH HHS/United States
- R01 NS031234/NS/NINDS NIH HHS/United States
- T32GM008362/GM/NIGMS NIH HHS/United States
- 1R01NS082851/NS/NINDS NIH HHS/United States
- F31 NS074597/NS/NINDS NIH HHS/United States
- P30 HD038985/HD/NICHD NIH HHS/United States
- T32 NS048039/NS/NINDS NIH HHS/United States
- P30 CA013148/CA/NCI NIH HHS/United States
- 2R01NS036692/NS/NINDS NIH HHS/United States
- R25 NS079188/NS/NINDS NIH HHS/United States
- 5R01NS052634/NS/NINDS NIH HHS/United States
- NS047466/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

