Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS
- PMID: 26019348
- PMCID: PMC4444552
- DOI: 10.1523/JNEUROSCI.2850-14.2015
Exclusion of integrins from CNS axons is regulated by Arf6 activation and the AIS
Abstract
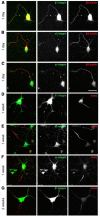
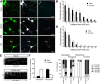
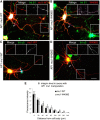
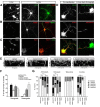
Integrins are adhesion and survival molecules involved in axon growth during CNS development, as well as axon regeneration after injury in the peripheral nervous system (PNS). Adult CNS axons do not regenerate after injury, partly due to a low intrinsic growth capacity. We have previously studied the role of integrins in axon growth in PNS axons; in the present study, we investigate whether integrin mechanisms involved in PNS regeneration may be altered or lacking from mature CNS axons by studying maturing CNS neurons in vitro. In rat cortical neurons, we find that integrins are present in axons during initial growth but later become restricted to the somato-dendritic domain. We investigated how this occurs and whether it can be altered to enhance axonal growth potential. We find a developmental change in integrin trafficking; transport becomes predominantly retrograde throughout axons, but not dendrites, as neurons mature. The directionality of transport is controlled through the activation state of ARF6, with developmental upregulation of the ARF6 GEF ARNO enhancing retrograde transport. Lowering ARF6 activity in mature neurons restores anterograde integrin flow, allows transport into axons, and increases axon growth. In addition, we found that the axon initial segment is partly responsible for exclusion of integrins and removal of this structure allows integrins into axons. Changing posttranslational modifications of tubulin with taxol also allows integrins into the proximal axon. The experiments suggest that the developmental loss of regenerative ability in CNS axons is due to exclusion of growth-related molecules due to changes in trafficking.
Keywords: Arf6; axon initial segment; axonal transport; integrin; trafficking.
Copyright © 2015 Franssen, Zhao et al.
Figures



References
-
- Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Kruppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
