Histochemical Analysis of Laminin α Chains in Diethylstilbestrol-Induced Prolactinoma in Rats
- PMID: 26019376
- PMCID: PMC4427567
- DOI: 10.1267/ahc.14067
Histochemical Analysis of Laminin α Chains in Diethylstilbestrol-Induced Prolactinoma in Rats
Abstract
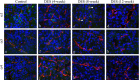
Laminin, a major basement membrane component, is important in structural support and cell proliferation and differentiation. Its 19 isoforms are assemblies of α, β, and γ chains, and the α chains (α1-5) determine the isoform characteristics. Although our previous studies showed alterations in α chain expressions during anterior pituitary development, their expressions in pituitary tumors yet to be determined. The present study used a rat model of diethylstilbestrol (DES)-induced prolactinoma to examine α chain expressions during prolactinoma tumorigenesis (0-12 weeks of DES treatment) by in situ hybridization and immunohistochemistry. mRNA of α1, α3, and α4 chains was detected in control and after 4 weeks of DES treatment. These expressions were undetectable after 8 weeks of DES treatment and in prolactinoma (12 weeks of DES treatment). Immunohistochemistry showed that the α1 chain was localized in some anterior pituitary cells in control and after 4 weeks of treatment and in endothelial cells after 8 weeks of treatment. The α3 and α4 chains were expressed in endothelial cells, and immunoreactivity and the number of immunopositive cells decreased during DES treatment. These findings suggest that alteration of laminin α chains is related to abnormal cell proliferation and neovascularization during development of DES-induced prolactinoma.
Keywords: anterior pituitary; diethylstilbestrol; laminin; prolactinoma; rat.
Figures

References
-
- Colognato-Pyke H., O’Rear J. J., Yamada Y., Carbonetto S., Cheng Y. S. and Yurchenco P. D. (1995) Mapping of network-forming, heparin-binding, and α1β1 integrin-recognition sites within the α-chain short arm of laminin-1. J. Biol. Chem. 270; 9398–9406. - PubMed
-
- Colognato H., MacCarrick M., O’Rear J. J. and Yurchenco P. D. (1997) The laminin α2-chain short arm mediates cell adhesion through both the α1β1 and α2β1 integrins. J. Biol. Chem. 272; 29330–29336. - PubMed
-
- Durbeej M. (2010) Laminins. Cell Tissue Res. 339; 259–268. - PubMed
-
- Fujiwara K., Davaadash B., Yatabe M., Kikuchi M., Horiguchi K., Kusumoto K., Kouki T. and Yashiro T. (2008) Reduction of retinaldehyde dehydrogenase 1 expression and production in estrogen-induced prolactinoma of rat. Med. Mol. Morphol. 41; 126–131. - PubMed
-
- Hogan B. L., Cooper A. R. and Kurkinen M. (1980) Incorporation into Reichert’s membrane of laminin-like extracellular proteins synthesized by parietal endoderm cells of the mouse embryo. Dev. Biol. 80; 289–300. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

