Inhibitor of caspase-activated DNase expression enhances caspase-activated DNase expression and inhibits oxidative stress-induced chromosome breaks at the mixed lineage leukaemia gene in nasopharyngeal carcinoma cells
- PMID: 26019688
- PMCID: PMC4446063
- DOI: 10.1186/s12935-015-0205-1
Inhibitor of caspase-activated DNase expression enhances caspase-activated DNase expression and inhibits oxidative stress-induced chromosome breaks at the mixed lineage leukaemia gene in nasopharyngeal carcinoma cells
Abstract
Background: Nasopharyngeal carcinoma (NPC) is commonly found in Asia, especially among the Chinese ethnic group. Chromosome rearrangements are common among NPC patients. Although the mechanism underlying the chromosome rearrangements in NPC is unclear, various mechanisms including activation of caspase-activated DNase (CAD) were proposed to contribute to chromosome rearrangements in leukaemia. Activation of CAD can be initiated by multiple agents, including oxidative stress, which is well implicated in carcinogenesis. CAD is the main enzyme that causes DNA fragmentation during apoptosis, and CAD is also implicated in promoting cell differentiation. In view of the role of oxidative stress in carcinogenesis and CAD activation, and since CAD was suggested to contribute to chromosome rearrangement in leukaemia, we hypothesise that oxidative stress-induced CAD activation could be one of the mechanisms that leads to chromosome rearrangements in NPC.
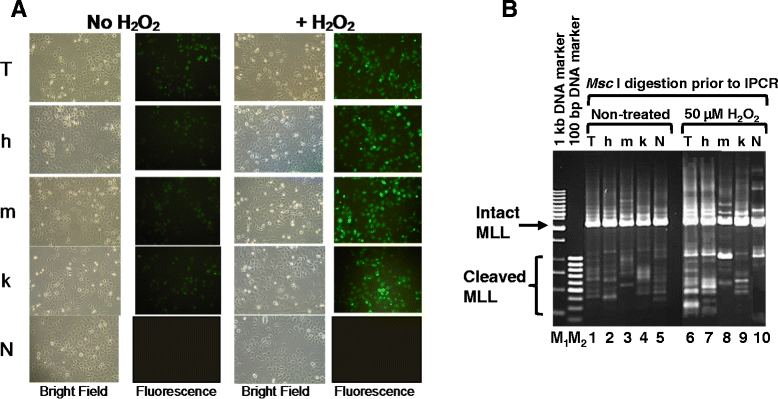
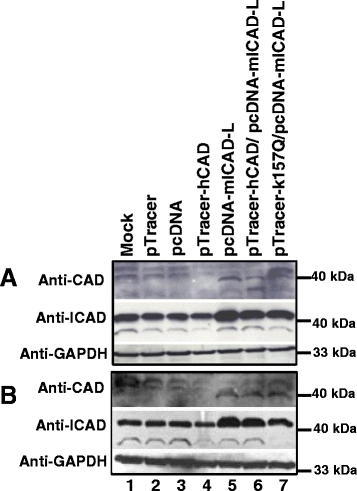
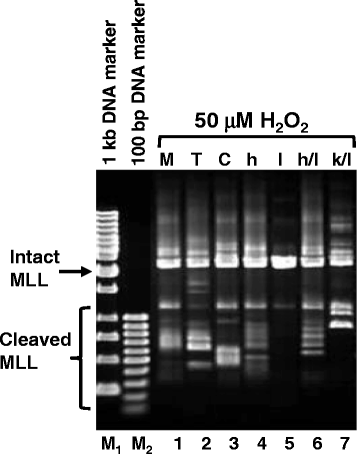
Methods: SUNEI cells were treated with various concentrations of H2O2 for different period of time to ensure that cells undergo H2O2-induced MLL gene cleavage. Transfections with hCAD, mCAD, mutant hCAD, or cotransfection with hCAD and mICAD, and cotransfection with mutant hCAD and mICAD were performed. Gene expression was confirmed by Western blotting and MLL gene cleavage was assessed by inverse polymerase chain reaction (IPCR).
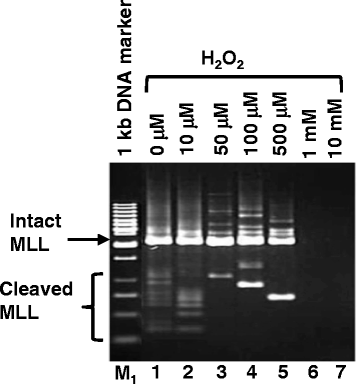
Results: Treatment with H2O2 clearly induces cleavages within the MLL gene which locates at 11q23, a common deletion site in NPC. In order to investigate the role of CAD, CAD was overexpressed in SUNE1 cells, but that did not result in significant changes in H2O2-induced MLL gene cleavage. This could be because CAD requires ICAD for proper folding. Indeed, by overexpressing ICAD alone or co-expressing ICAD with CAD, Western blotting showed that CAD was expressed. In addition, ICAD overexpression also suppressed H2O2-induced MLL gene cleavage, suggesting a possible role of CAD in initiating chromosome cleavage during oxidative stress.
Conclusions: Oxidative stress mediated by H2O2 induces cleavage of the MLL gene, most likely via the caspase-activated DNase, CAD, and CAD expression requires ICAD. Since the MLL gene is located at 11q23, a common deletion site in NPC, thus stress-induced CAD activation may represent one of the mechanisms leading to chromosome rearrangement in NPC.
Keywords: CAD; Chromosome breaks; ICAD; MLL; Nasopharyngeal carcinoma; Oxidative stress.
Figures

References
-
- Fandi A, Altun M, Azli N, Armand JP, Cvitkovic E. Nasopharyngeal cancer: epidemiology, staging, and treatment. Semin Oncol. 1994;21:382–97. - PubMed
-
- Voravud N. Cancer in the far East. In: Sikora K, HKE, editor. Treatment of cancer. London: Chapman and Hall Medical; 1990. pp. 887–94.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

