Calcium affinity of human α-actinin 1
- PMID: 26020004
- PMCID: PMC4435478
- DOI: 10.7717/peerj.944
Calcium affinity of human α-actinin 1
Abstract
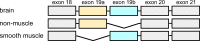
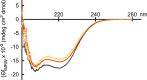
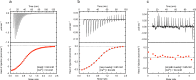
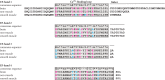
Due to alternative splicing, the human ACTN1 gene codes for three different transcripts of α-actinin; one isoform that is expressed only in the brain and two with a more general expression pattern. The sequence difference is located to the C-terminal domains and the EF-hand motifs. Therefore, any functional or structural distinction should involve this part of the protein. To investigate this further, the calcium affinities of these three isoforms of α-actinin 1 have been determined by isothermal calorimetry.
Keywords: EF-hand; calcium binding; α-actinin.
Conflict of interest statement
The authors declare there are no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

