Macrophage Migration Inhibitory Factor Mediates PAR-Induced Bladder Pain
- PMID: 26020638
- PMCID: PMC4447427
- DOI: 10.1371/journal.pone.0127628
Macrophage Migration Inhibitory Factor Mediates PAR-Induced Bladder Pain
Abstract
Introduction: Macrophage migration inhibitory factor (MIF), a pro-inflammatory cytokine, is constitutively expressed in urothelial cells that also express protease-activated receptors (PAR). Urothelial PAR1 receptors were shown to mediate bladder inflammation. We showed that PAR1 and PAR4 activator, thrombin, also mediates urothelial MIF release. We hypothesized that stimulation of urothelial PAR1 or PAR4 receptors elicits release of urothelial MIF that acts on MIF receptors in the urothelium to mediate bladder inflammation and pain. Thus, we examined the effect of activation of specific bladder PAR receptors on MIF release, bladder pain, micturition and histological changes.
Methods: MIF release was measured in vitro after exposing immortalized human urothelial cells (UROtsa) to PAR1 or PAR4 activating peptides (AP). Female C57BL/6 mice received intravesical PAR1- or PAR4-AP for one hour to determine: 1) bladder MIF release in vivo within one hour; 2) abdominal hypersensitivity (allodynia) to von Frey filament stimulation 24 hours after treatment; 3) micturition parameters 24 hours after treatment; 4) histological changes in the bladder as a result of treatment; 5) changes in expression of bladder MIF and MIF receptors using real-time RT-PCR; 6) changes in urothelial MIF and MIF receptor, CXCR4, protein levels using quantitative immunofluorescence; 7) effect of MIF or CXCR4 antagonism.
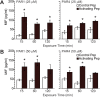
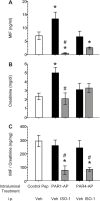
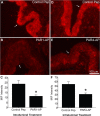
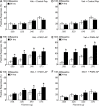
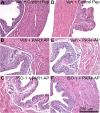
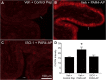
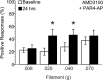
Results: PAR1- or PAR4-AP triggered MIF release from both human urothelial cells in vitro and mouse urothelium in vivo. Twenty-four hours after intravesical PAR1- or PAR4-AP, we observed abdominal hypersensitivity in mice without changes in micturition or bladder histology. PAR4-AP was more effective and also increased expression of bladder MIF and urothelium MIF receptor, CXCR4. Bladder CXCR4 localized to the urothelium. Antagonizing MIF with ISO-1 eliminated PAR4- and reduced PAR1-induced hypersensitivity, while antagonizing CXCR4 with AMD3100 only partially prevented PAR4-induced hypersensitivity.
Conclusions: Bladder PAR activation elicits urothelial MIF release and urothelial MIF receptor signaling at least partly through CXCR4 to result in abdominal hypersensitivity without overt bladder inflammation. PAR-induced bladder pain may represent an interesting pre-clinical model of Interstitial Cystitis/Painful Bladder Syndrome (IC/PBS) where pain occurs without apparent bladder injury or pathology. MIF is potentially a novel therapeutic target for bladder pain in IC/PBS patients.
Conflict of interest statement
Figures
References
-
- Hoi AY, Iskander MN, Morand EF. Macrophage migration inhibitory factor: a therapeutic target across inflammatory diseases. Inflamm Allergy Drug Targets. 2007;6(3):183–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

