Bacterial spread from cell to cell: beyond actin-based motility
- PMID: 26021574
- PMCID: PMC4560970
- DOI: 10.1016/j.tim.2015.04.010
Bacterial spread from cell to cell: beyond actin-based motility
Abstract
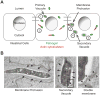
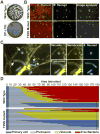
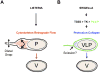
Several intracellular pathogens display the ability to propagate within host tissues by displaying actin-based motility in the cytosol of infected cells. As motile bacteria reach cell-cell contacts they form plasma membrane protrusions that project into adjacent cells and resolve into vacuoles from which the pathogen escapes, thereby achieving spread from cell to cell. Seminal studies have defined the bacterial and cellular factors that support actin-based motility. By contrast, the mechanisms supporting the formation of protrusions and their resolution into vacuoles have remained elusive. Here, we review recent advances in the field showing that Listeria monocytogenes and Shigella flexneri have evolved pathogen-specific mechanisms of bacterial spread from cell to cell.
Keywords: Listeria monocytogenes; Shigella flexneri; double-membrane vacuole; membrane protrusion; spread from cell to cell.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


References
-
- Gouin E, Gantelet H, Egile C, Lasa I, Ohayon H, et al. A comparative study of the actin-based motilities of the pathogenic bacteria Listeria monocytogenes, Shigella flexneri and Rickettsia conorii. J Cell Sci. 1999;112(Pt 11):1697–1708. - PubMed
-
- Monack DM, Theriot JA. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell Microbiol. 2001;3:633–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

