Serotonin Attenuates Feedback Excitation onto O-LM Interneurons
- PMID: 26021702
- PMCID: PMC4816800
- DOI: 10.1093/cercor/bhv098
Serotonin Attenuates Feedback Excitation onto O-LM Interneurons
Abstract
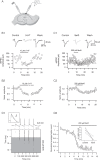
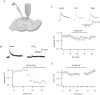
The serotonergic system is a subcortical neuromodulatory center that controls cortical information processing in a state-dependent manner. In the hippocampus, serotonin (5-HT) is released by ascending serotonergic fibers from the midbrain raphe nuclei, thereby mediating numerous modulatory functions on various neuronal subtypes. Here, we focus on the neuromodulatory effects of 5-HT on GABAergic inhibitory oriens lacunosum-moleculare (O-LM) cells in the hippocampal area CA1 of the rat. These interneurons are thought to receive primarily local excitatory input and are, via their axonal projections to stratum lacunosum-moleculare, ideally suited to control entorhinal cortex input. We show that 5-HT reduces excitatory glutamatergic transmission onto O-LM interneurons. By means of paired recordings from synaptically connected CA1 pyramidal cells and O-LM interneurons we reveal that this synapse is modulated by 5-HT. Furthermore, we demonstrate that the reduction of glutamatergic transmission by serotonin is likely to be mediated via a decrease of calcium influx into presynaptic terminals of CA1 pyramidal cells. This modulation of excitatory synaptic transmission onto O-LM interneurons by 5-HT might be a mechanism to vary the activation of O-LM interneurons during ongoing network activity and serve as a brain state-dependent switch gating the efficiency of entorhinal cortex input to CA1 pyramidal neurons.
Keywords: 5-HT; CA1; paired recordings; presynaptic neuromodulation.
© The Author 2015. Published by Oxford University Press.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

