Deaths Reported to the Vaccine Adverse Event Reporting System, United States, 1997-2013
- PMID: 26021988
- PMCID: PMC6771280
- DOI: 10.1093/cid/civ423
Deaths Reported to the Vaccine Adverse Event Reporting System, United States, 1997-2013
Abstract
Background: Vaccines are among the safest medical products in use today. Hundreds of millions of vaccinations are administered in the United States each year. Serious adverse reactions are uncommon. However, temporally associated deaths can occur following vaccination. Our aim was to characterize main causes of death among reports submitted to the US Vaccine Adverse Event Reporting System (VAERS), a spontaneous vaccine safety surveillance system.
Methods: We searched VAERS for US reports of death after any vaccination from 1 July 1997 through 31 December 2013. Available medical records, autopsy reports, and death certificates were reviewed to identify cause of death.
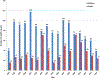
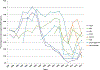
Results: VAERS received 2149 death reports, most (n = 1469 [68.4%]) in children. Median age was 0.5 years (range, 0-100 years); males accounted for 1226 (57%) reports. The total annual number of death reports generally decreased during the latter part of the study period. Most common causes of death among 1244 child reports with available death certificates/autopsy reports included sudden infant death syndrome (n = 544 [44%]), asphyxia (n = 74 [6.0%]), septicemia (n = 61 [4.9%]), and pneumonia (n = 57 [4.6%]). Among 526 adult reports, most common causes of death included diseases of the circulatory (n = 247 [46.9%]) and respiratory systems (n = 77 [14.6%]), certain infections and parasitic diseases (n = 62 [11.8%]), and malignant neoplasms (n = 20 [3.8%]). For child death reports, 79.4% received >1 vaccine on the same day. Inactivated influenza vaccine given alone was most commonly associated with death reports in adults (51.4%).
Conclusions: No concerning pattern was noted among death reports submitted to VAERS during 1997-2013. The main causes of death were consistent with the most common causes of death in the US population.
Keywords: death; epidemiology; surveillance; vaccine safety; vaccines.
Published by Oxford University Press on behalf of the Infectious Diseases Society of America 2015. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Conflict of interest statement
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Nakada H, Narimatsu H, Tsubokura M, et al. Risk of fatal adverse events after H1N1 influenza vaccination. Clin Infect Dis 2010; 50:1548–9. - PubMed
-
- McNeil MM, Broder KR, Vellozzi C, DeStefano F. Risk of fatal adverse events after H1N1 influenza vaccine: limitations of passive surveillance data. Clin Infect Dis 2010; 51:871–2. - PubMed
-
- Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare of Japan. Safety review results of pneumococcal conjugate vaccine and Haemophilus influenzae type b (Hib) vaccine for pediatrics, 2011. Available at: http://www.pmda.go.jp/files/000153722.pdf Accessed 5 June 2015.
-
- Pharmaceutical and Food Safety Bureau, Ministry of Health, Labour and Welfare of Japan. Pharmaceuticals and medical devices safety information No. 280, 2011. Available at: https://www.pmda.go.jp/files/000153635.pdf Accessed 5 June 2015.
-
- Global Advisory Committee on Vaccine Safety, 12–13 June 2013. Wkly Epidemiol Rec 2013; 88:301–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

